In response to protein load podocytes reorganize cytoskeleton and modulate endothelin-1 gene: implication for permselective dysfunction of chronic nephropathies
- PMID: 15855633
- PMCID: PMC1606387
- DOI: 10.1016/S0002-9440(10)62350-4
In response to protein load podocytes reorganize cytoskeleton and modulate endothelin-1 gene: implication for permselective dysfunction of chronic nephropathies
Abstract
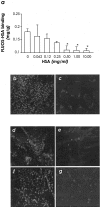
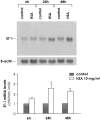
Effacement of podocyte foot processes occurs in many proteinuric nephropathies and is accompanied by rearrangement of the actin cytoskeleton. Here, we studied whether protein overload affects intracellular pathways, leading to cytoskeletal architecture changes and ultimately to podocyte dysfunction. Mouse podocytes bound and endocytosed both albumin and IgG via receptor-specific mechanisms. Protein overload caused redistribution of F-actin fibers instrumental to up-regulation of the prepro-endothelin (ET)-1 gene and production of the corresponding peptide. Increased DNA-binding activity for nuclear factor (NF)-kappaB and Ap-1 nuclear proteins was measured in nuclear extracts of podocytes exposed to excess proteins. Both Y27632, which inhibits Rho kinase-dependent stress fiber formation, and jasplakinolide, an F-actin stabilizer, decreased NF-kappaB and Ap-1 activity and reduced ET-1 expression. This suggested a role for the cytoskeleton, through activated Rho, in the regulation of the ET-1 peptide. Focal adhesion kinase (FAK), an integrin-associated nonreceptor tyrosine kinase, was phosphorylated by albumin treatment via Rho kinase-triggered actin reorganization. FAK activation led to NF-kappaB- and Ap-1-dependent ET-1 expression. These data suggest that reorganization of the actin cytoskeletal network in response to protein load is implicated in modulation of the ET-1 gene via Rho kinase-dependent FAK activation of NF-kappaB and Ap-1 in differentiated podocytes. Increased ET-1 generation might alter glomerular permselectivity and amplify the noxious effect of protein overload on dysfunctional podocytes.
Figures



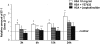

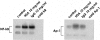



References
-
- Olson JL, Hostetter TH, Rennke HG, Brenner BM, Venkatachalam MA. Altered glomerular permselectivity and progressive sclerosis following extreme ablation of renal mass. Kidney Int. 1982;22:112–126. - PubMed
-
- Brenner BM, Meyer TW, Hostetter TH. Dietary protein intake and progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med. 1982;307:652–659. - PubMed
-
- Fries JW, Sandstrom DJ, Meyer TW, Rennke HG. Glomerular hypertrophy and epithelial cell injury modulate progressive glomerulosclerosis in the rat. Lab Invest. 1989;60:205–218. - PubMed
-
- Remuzzi G, Bertani T. Is glomerulosclerosis a consequence of altered glomerular permeability to macromolecules? Kidney Int. 1990;38:384–394. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

