Absence of proteinase-activated receptor-1 signaling affords protection from bleomycin-induced lung inflammation and fibrosis
- PMID: 15855637
- PMCID: PMC1606391
- DOI: 10.1016/S0002-9440(10)62354-1
Absence of proteinase-activated receptor-1 signaling affords protection from bleomycin-induced lung inflammation and fibrosis
Abstract
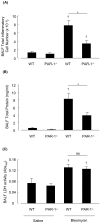
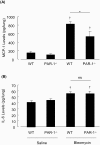
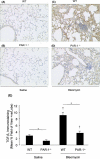
Activation of the coagulation cascade is commonly observed in the lungs of patients with both acute and chronic inflammatory and fibrotic lung disorders, as well as in animal models of these disorders. The aim of this study was to examine the contribution of the major thrombin receptor, proteinase-activated receptor-1 (PAR-1), during the acute inflammatory and chronic fibrotic phases of lung injury induced by intratracheal instillation of bleomycin in mice. Inflammatory cell recruitment and increases in bronchoalveolar lavage fluid (BALF) protein were attenuated by 56 +/- 10% (P < 0.05) and 53 +/- 12% (P < 0.05), respectively, in PAR-1-deficient (PAR-1-/-) mice compared with wild-type (WT) mice. PAR-1-/- mice were also protected from bleomycin-induced pulmonary fibrosis with total lung collagen accumulation reduced by 59 +/- 5% (P < 0.05). The protection afforded by PAR-1 deficiency was accompanied by significant reductions in pulmonary levels of the potent PAR-1-inducible proinflammatory and profibrotic mediators, monocyte chemoattractant protein-1 (MCP-1), transforming growth factor-beta-1 (TGF-beta1), and connective tissue growth factor/fibroblast-inducible secreted protein-12 (CTGF/FISP12). In addition, PAR-1 was highly expressed in inflammatory and fibroproliferative lesions in lung sections obtained from patients with fibrotic lung disease. These data show for the first time that PAR-1 signaling plays a key role in experimentally induced lung injury, and they further identify PAR-1 as one of the critical receptors involved in orchestrating the interplay between coagulation, inflammation, and remodeling in response to tissue injury.
Figures




References
-
- Chambers RC, Laurent GJ. Coagulation cascade proteases and tissue fibrosis. Biochem Soc Trans. 2002;30:194–200. - PubMed
-
- Idell S, Gonzalez K, Bradford H, MacArthur CK, Fein AM, Maunder RJ, Garcia JG, Griffith DE, Weiland J, Martin TR, McLarty J, Fair DS, Walsh PN, Colman RW. Procoagulant activity in bronchoalveolar lavage in the adult respiratory distress syndrome: contribution of tissue factor associated with factor VII. Am Rev Respir Dis. 1987;136:1466–1474. - PubMed
-
- Imokawa S, Sato A, Hayakawa H, Kotani M, Urano T, Takada A. Tissue factor expression and fibrin deposition in the lungs of patients with idiopathic pulmonary fibrosis and systemic sclerosis. Am J Respir Crit Care Med. 1997;156:631–636. - PubMed
-
- Idell S, Mazar AP, Bitterman P, Mohla S, Harabin AL. Fibrin turnover in lung inflammation and neoplasia. Am J Respir Crit Care Med. 2001;163:578–584. - PubMed
-
- Gunther A, Lubke N, Ermert M, Schermuly RT, Weissmann N, Breithecker A, Markart P, Ruppert C, Quanz K, Ermert L, Grimminger F, Seeger W. Prevention of bleomycin-induced lung fibrosis by aerosolization of heparin or urokinase in rabbits. Am J Respir Crit Care Med. 2003;168:1358–1365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

