Platelet-activating factor regulates cadherin-catenin adhesion system expression and beta-catenin phosphorylation during Kaposi's sarcoma cell motility
- PMID: 15855650
- PMCID: PMC1620029
- DOI: 10.1016/s0002-9440(10)62367-x
Platelet-activating factor regulates cadherin-catenin adhesion system expression and beta-catenin phosphorylation during Kaposi's sarcoma cell motility
Abstract
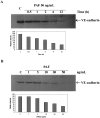
In the present study, we evaluated whether motility of Kaposi's sarcoma (KS) cells induced by platelet-activating factor (PAF) is dependent on the regulation of adherens junctions components. The results obtained indicate that PAF dose and time dependently reduced the endogenous expression of the main components of the adherens junctions: VE-cadherin, alpha-catenin, and beta-catenin. In addition, PAF initiated events that directly or indirectly up-regulated both the tyrosine and serine/threonine phosphorylation pathways, and both types of phosphorylation of beta-catenin were involved in the motility of KS cells. This motility was abrogated by addition of the tyrosine kinase inhibitor genistein, suggesting that this phosphorylation is an important signal responsible for breaking down the adherens junctions and diminishing the ability of neighboring cells to interact. Furthermore, immunofluorescence analysis showed that beta-catenin and VE-cadherin staining changed from a uniform distribution along the membrane of controls to a diffuse pattern with gap formation in PAF-treated KS cells. In conclusion, the data presented here indicate that PAF induces tumor cell motility by altering cell-cell adhesion through beta-catenin phosphorylation.
Figures




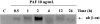


References
-
- Prescott SM, Zimmerman GA, McIntyre TM. Platelet-activating factor. J Biol Chem. 1990;265:17381–17384. - PubMed
-
- Kume K, Shimizu T. Platelet-activating factor (PAF) induces growth stimulation, inhibition, and suppression of oncogenic transformation in NRK cells overexpressing the PAF receptor. J Biol Chem. 1997;272:22898–22904. - PubMed
-
- Boccellino M, Biancone L, Cantaluppi V, Ye RD, Camussi G. Effect of platelet-activating factor receptor expression on CHO cell motility. J Cell Physiol. 2000;183:254–264. - PubMed
-
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

