Up-regulated expression of zonula occludens protein-1 in human melanoma associates with N-cadherin and contributes to invasion and adhesion
- PMID: 15855653
- PMCID: PMC1606406
- DOI: 10.1016/S0002-9440(10)62370-X
Up-regulated expression of zonula occludens protein-1 in human melanoma associates with N-cadherin and contributes to invasion and adhesion
Abstract
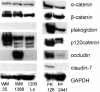
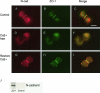
During the process of malignant transformation, nascent melanoma cells escape keratinocyte control through down-regulation of E-cadherin and instead communicate among themselves and with fibroblasts via N-cadherin-based cell-cell contacts. The zonula occludens (ZO) protein-1 is a membrane-associated component of both the tight and adherens junctions found at sites of cell-cell contact. In most cancers, levels of ZO-1 are typically down-regulated, leading to increased motility. Here we report the novel observation that ZO-1 expression is up-regulated in melanoma cells and is located at adherens junctions between melanoma cells and fibroblasts. Immunofluorescence and co-immunoprecipitation studies showed co-localization of ZO-1 with N-cadherin. Down-regulation of ZO-1 in melanoma cells through RNA interference produced marked changes in cell morphology--leading to a less-dendritic, more rounded phenotype. Consistent with a role in N-cadherin-based adhesion, RNAi-treated melanoma cells were less adherent and invasive when grown in a collagen gel. These data provide the first evidence that increased ZO-1 expression in melanoma contributes to the oncogenic behavior of this tumor and further illustrate that protein products of genes, such as ZO-1, can function in either a pro- or anti-oncogenic manner when expressed in different cellular contexts.
Figures







References
-
- Tang A, Eller MS, Hara M, Yaar M, Hirohashi S, Gilchrest BA. E-cadherin is the major mediator of human melanocyte adhesion to keratinocytes in vitro. J Cell Sci. 1994;107:983–992. - PubMed
-
- Hsu MY, Andl T, Li G, Meinkoth JL, Herlyn M. Cadherin repertoire determines partner-specific gap junctional communication during melanoma progression. J Cell Sci. 2000;113:1535–1542. - PubMed
-
- Haass NK, Smalley KSM, Herlyn M. The role of altered cell-cell communication in melanoma progression. J Mol Histol. 2004;35:309–318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

