Loss of TGF-beta type II receptor in fibroblasts promotes mammary carcinoma growth and invasion through upregulation of TGF-alpha-, MSP- and HGF-mediated signaling networks
- PMID: 15856015
- PMCID: PMC3074577
- DOI: 10.1038/sj.onc.1208685
Loss of TGF-beta type II receptor in fibroblasts promotes mammary carcinoma growth and invasion through upregulation of TGF-alpha-, MSP- and HGF-mediated signaling networks
Abstract
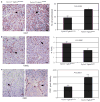
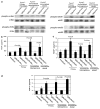
Stromal fibroblasts regulate epithelial cell behavior through direct and indirect cell-cell interactions. To clarify the role of TGF-beta signaling in stromal fibroblasts during mammary development and tumorigenesis, we conditionally knocked out the TGF-beta type II receptor gene in mouse mammary fibroblasts (Tgfbr2(fspKO)). Tgfbr2(fspKO) mice exhibit defective mammary ductal development, characterized in part by increased ductal epithelial cell turnover associated with an increase in stromal fibroblast abundance. Tgfbr2(fspKO) mammary fibroblasts transplanted with mammary carcinoma cells promote growth and invasion, which is associated with increased activating phosphorylation of the receptors: erbB1, erbB2, RON, and c-Met. Furthermore, the increased receptor phosphorylation correlates with increased secretion of the cognate ligands by Tgfbr2(fspKO) fibroblasts. Treatment of tumor cells with fibroblast-conditioned medium leads to increased tumor cell proliferation and motility, which are blocked by addition of pharmacologic inhibitors of TGF-alpha signaling or neutralizing antibodies to macrophage-stimulating protein (MSP), HGF, or c-Met. These studies characterize a significant role for stromal TGF-beta signaling in mammary tissue homeostasis and mammary tumor progression via regulation of TGF-alpha, MSP, and HGF signaling pathways.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

