Antiangiogenic chimeric anti-endoglin (CD105) antibody: pharmacokinetics and immunogenicity in nonhuman primates and effects of doxorubicin
- PMID: 15856228
- PMCID: PMC11030172
- DOI: 10.1007/s00262-005-0691-4
Antiangiogenic chimeric anti-endoglin (CD105) antibody: pharmacokinetics and immunogenicity in nonhuman primates and effects of doxorubicin
Abstract
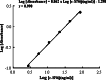
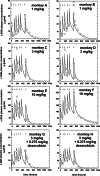
We generated a human/mouse chimeric antibody c-SN6j of human IgG1 isotype from a murine anti-human endoglin (EDG) monoclonal antibody (mAb) SN6j that suppressed angiogenesis, tumor growth and metastasis in mice. We determined pharmacokinetics (PKs) and immunogenicity of c-SN6j in monkeys after multiple i.v. injections. A dose-escalation study was performed by administration of c-SN6j into six monkeys at the dose of 1 mg, 3 mg and 10 mg per kg body weight. In addition, both c-SN6j (3 mg/kg) and doxorubicin (0.275 mg/kg) were injected into two monkeys. c-SN6j and doxorubicin were injected twice a week for 3 weeks. We developed a unique and sensitive ELISA by sequentially targeting the common and idiotypic epitopes of c-SN6j-Fv to quantify plasma c-SN6j. Application of the ELISA showed that increasing the c-SN6j dose resulted in a proportional increase in the circulating c-SN6j after the first injection. In addition, the estimated area under the curve (AUC) for the first injection of c-SN6j is proportional to dose. We carried out detailed analyses of PKs of c-SN6j during and after the repeated injections. Our model of PKs fitted the empirical data well. Addition of doxorubicin modulated the PK parameters. We developed two ELISAs to separately determine the immune responses to the murine part and the human part of c-SN6j in monkeys. Interestingly, the murine part induced a weaker immune response than the human part. Doxorubicin potentiated the immune responses. Increasing the dose of c-SN6j increased plasma levels of c-SN6j but did not increase the immune responses to c-SN6j.
Figures


References
-
- Gougos A, Letarte M. Identification of a human endothelial cell antigen with monoclonal antibody 44G4 produced against a pre-B leukemic cell line. J Immunol. 1988;141:1925–1933. - PubMed
-
- Gougos A, Letarte M. Primary structure of endoglin, an RGD-containing glycoprotein of human endothelial cells. J Biol Chem. 1990;265:8361–8364. - PubMed
-
- Bellon T, Corbi A, Lastres P, Cales C, Cerbrian M, Vera S, Cheifetz S, Massague J, Letarte M, Bernabeu C. Identification and expression of two forms of the human transforming growth factor-beta-binding protein endoglin with distinct cytoplasmic regions. Eur J Immunol. 1993;23:2340–2345. doi: 10.1002/eji.1830230943. - DOI - PubMed
-
- Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J, Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992;267:19027–19030. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

