Output-mode transitions are controlled by prolonged inactivation of sodium channels in pyramidal neurons of subiculum
- PMID: 15857153
- PMCID: PMC1088280
- DOI: 10.1371/journal.pbio.0030175
Output-mode transitions are controlled by prolonged inactivation of sodium channels in pyramidal neurons of subiculum
Abstract
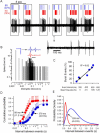
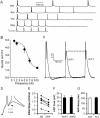
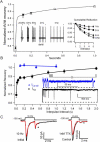
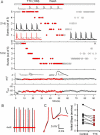
Transitions between different behavioral states, such as sleep or wakefulness, quiescence or attentiveness, occur in part through transitions from action potential bursting to single spiking. Cortical activity, for example, is determined in large part by the spike output mode from the thalamus, which is controlled by the gating of low-voltage-activated calcium channels. In the subiculum--the major output of the hippocampus--transitions occur from bursting in the delta-frequency band to single spiking in the theta-frequency band. We show here that these transitions are influenced strongly by the inactivation kinetics of voltage-gated sodium channels. Prolonged inactivation of sodium channels is responsible for an activity-dependent switch from bursting to single spiking, constituting a novel mechanism through which network dynamics are controlled by ion channel gating.
Figures
References
-
- Sherman SM. Tonic and burst firing: Dual modes of thalamocortical relay. Trends Neurosci. 2001;24:122–126. - PubMed
-
- Sherman SM, Guillery RW. Functional organization of thalamocortical relays. J Neurophysiol. 1996;76:1367–1395. - PubMed
-
- Weyand TG, Boudreaux M, Guido W. Burst and tonic response modes in thalamic neurons during sleep and wakefulness. J Neurophysiol. 2001;85:1107–1118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

