Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells
- PMID: 15857154
- PMCID: PMC1088276
- DOI: 10.1371/journal.pbio.0030150
Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells
Abstract
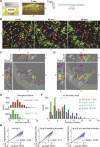
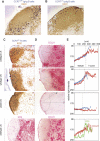
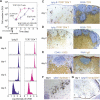
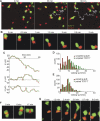
Interactions between B and T cells are essential for most antibody responses, but the dynamics of these interactions are poorly understood. By two-photon microscopy of intact lymph nodes, we show that upon exposure to antigen, B cells migrate with directional preference toward the B-zone-T-zone boundary in a CCR7-dependent manner, through a region that exhibits a CCR7-ligand gradient. Initially the B cells show reduced motility, but after 1 d, motility is increased to approximately 9 microm/min. Antigen-engaged B cells pair with antigen-specific helper T cells for 10 to more than 60 min, whereas non-antigen-specific interactions last less than 10 min. B cell-T cell conjugates are highly dynamic and migrate extensively, being led by B cells. B cells occasionally contact more than one T cell, whereas T cells are strictly monogamous in their interactions. These findings provide evidence of lymphocyte chemotaxis in vivo, and they begin to define the spatiotemporal cellular dynamics associated with T cell-dependent antibody responses.
Figures



References
-
- MacLennan IC, Gulbranson-Judge A, Toellner KM, Casamayor-Palleja M, Chan E, et al. The changing preference of T and B cells for partners as T-dependent antibody responses develop. Immunol Rev. 1997;156:53–66. - PubMed
-
- Mills DM, Cambier JC. B lymphocyte activation during cognate interactions with CD4+ T lymphocytes: Molecular dynamics and immunologic consequences. Semin Immunol. 2003;15:325–329. - PubMed
-
- Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. - PubMed
-
- Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan IC. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991;21:2951–2962. - PubMed
-
- Cyster JG. Signaling thresholds and interclonal competition in preimmune B-cell selection. Immunol Rev. 1997;156:87–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

