Sensitization and translocation of TRPV1 by insulin and IGF-I
- PMID: 15857517
- PMCID: PMC1142339
- DOI: 10.1186/1744-8069-1-17
Sensitization and translocation of TRPV1 by insulin and IGF-I
Abstract
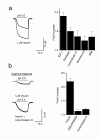
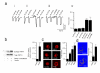
Insulin and insulin-like growth factors (IGFs) maintain vital neuronal functions. Absolute or functional deficiencies of insulin or IGF-I may contribute to neuronal and vascular complications associated with diabetes. Vanilloid receptor 1 (also called TRPV1) is an ion channel that mediates inflammatory thermal nociception and is present on sensory neurons. Here we demonstrate that both insulin and IGF-I enhance TRPV1-mediated membrane currents in heterologous expression systems and cultured dorsal root ganglion neurons. Enhancement of membrane current results from both increased sensitivity of the receptor and translocation of TRPV1 from cytosol to plasma membrane. Receptor tyrosine kinases trigger a signaling cascade leading to activation of phosphatidylinositol 3-kinase (PI(3)K) and protein kinase C (PKC)-mediated phosphorylation of TRPV1, which is found to be essential for the potentiation. These findings establish a link between the insulin family of trophic factors and vanilloid receptors.
Figures
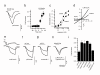

Similar articles
-
Insulin and insulin-like growth factor type-I up-regulate the vanilloid receptor-1 (TRPV1) in stably TRPV1-expressing SH-SY5Y neuroblastoma cells.J Neurosci Res. 2007 May 15;85(7):1413-9. doi: 10.1002/jnr.21255. J Neurosci Res. 2007. PMID: 17385724
-
Differential contribution of SNARE-dependent exocytosis to inflammatory potentiation of TRPV1 in nociceptors.FASEB J. 2009 Nov;23(11):3722-33. doi: 10.1096/fj.09-134346. Epub 2009 Jul 7. FASEB J. 2009. PMID: 19584302
-
Enhanced function of TRPV1 via up-regulation by insulin-like growth factor-1 in a rat model of bone cancer pain.Eur J Pain. 2014 Jul;18(6):774-84. doi: 10.1002/j.1532-2149.2013.00420.x. Epub 2013 Oct 29. Eur J Pain. 2014. PMID: 24827675
-
Insulin and IGF-1 signaling in oocyte maturation.Horm Res. 1994;42(1-2):55-61. doi: 10.1159/000184146. Horm Res. 1994. PMID: 7959635 Review.
-
New Mechanism of Bone Cancer Pain: Tumor Tissue-Derived Endogenous Formaldehyde Induced Bone Cancer Pain via TRPV1 Activation.Adv Exp Med Biol. 2016;904:41-58. doi: 10.1007/978-94-017-7537-3_4. Adv Exp Med Biol. 2016. PMID: 26900062 Review.
Cited by
-
Increased expression of Trpv1 in peripheral terminals mediates thermal nociception in Fabry disease mouse model.Mol Pain. 2016 Aug 16;12:1744806916663729. doi: 10.1177/1744806916663729. Print 2016. Mol Pain. 2016. PMID: 27531673 Free PMC article.
-
Tumour-Derived Glutamate: Linking Aberrant Cancer Cell Metabolism to Peripheral Sensory Pain Pathways.Curr Neuropharmacol. 2017;15(4):620-636. doi: 10.2174/1570159X14666160509123042. Curr Neuropharmacol. 2017. PMID: 27157265 Free PMC article.
-
Unusual localization and translocation of TRPV4 protein in cultured ventricular myocytes of the neonatal rat.Eur J Histochem. 2012 Jul 24;56(3):e32. doi: 10.4081/ejh.2012.e32. Eur J Histochem. 2012. PMID: 23027348 Free PMC article.
-
The mu opioid agonist morphine modulates potentiation of capsaicin-evoked TRPV1 responses through a cyclic AMP-dependent protein kinase A pathway.Mol Pain. 2006 Jul 16;2:22. doi: 10.1186/1744-8069-2-22. Mol Pain. 2006. PMID: 16842630 Free PMC article.
-
TRP Channels in the Focus of Trigeminal Nociceptor Sensitization Contributing to Primary Headaches.Int J Mol Sci. 2020 Jan 4;21(1):342. doi: 10.3390/ijms21010342. Int J Mol Sci. 2020. PMID: 31948011 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

