Receptor-independent spread of a highly neurotropic murine coronavirus JHMV strain from initially infected microglial cells in mixed neural cultures
- PMID: 15857995
- PMCID: PMC1091713
- DOI: 10.1128/JVI.79.10.6102-6110.2005
Receptor-independent spread of a highly neurotropic murine coronavirus JHMV strain from initially infected microglial cells in mixed neural cultures
Abstract
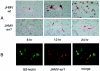
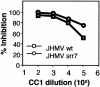
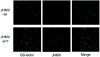
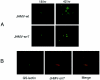
Although neurovirulent mouse hepatitis virus (MHV) strain JHMV multiplies in a variety of brain cells, expression of its receptor carcinoembryonic antigen cell adhesion molecule 1 (CEACAM 1) (MHVR) is restricted only in microglia. The present study was undertaken to clarify the mechanism of an extensive JHMV infection in the brain by using neural cells isolated from mouse brain. In contrast to wild-type (wt) JHMV, a soluble-receptor-resistant mutant (srr7) infects and spreads solely in an MHVR-dependent fashion (F. Taguchi and S. Matsuyama, J. Virol. 76:950-958, 2002). In mixed neural cell cultures, srr7 infected a limited number of cells and infection did not spread, although wt JHMV induced syncytia in most of the cells. srr7-infected cells were positive for GS-lectin, a microglia marker. Fluorescence-activated cell sorter analysis showed that about 80% of the brain cells stained with anti-MHVR antibody (CC1) were also positive for GS-lectin. Pretreatment of those cells with CC1 prevented virus attachment to the cell surface and also blocked virus infection. These results show that microglia express functional MHVR that mediates JHMV infection. As expected, in microglial cell-enriched cultures, both srr7and wt JHMV produced syncytia in a majority of cells. Treatment with CC1 of mixed neural cell cultures and microglia cultures previously infected with wt virus failed to block the spread of infection, indicating that wt infection spreads in an MHVR-independent fashion. Thus, the present study indicates that microglial cells are the major population of the initial target for MHV infection and that the wt spreads from initially infected microglia to a variety of cells in an MHVR-independent fashion.
Figures



Similar articles
-
Receptor-independent infection of murine coronavirus: analysis by spinoculation.J Virol. 2006 May;80(10):4901-8. doi: 10.1128/JVI.80.10.4901-4908.2006. J Virol. 2006. PMID: 16641281 Free PMC article.
-
Soluble receptor potentiates receptor-independent infection by murine coronavirus.J Virol. 2002 Feb;76(3):950-8. doi: 10.1128/jvi.76.3.950-958.2002. J Virol. 2002. PMID: 11773370 Free PMC article.
-
Heparan sulfate is a binding molecule but not a receptor for CEACAM1-independent infection of murine coronavirus.Virology. 2007 Sep 15;366(1):16-22. doi: 10.1016/j.virol.2007.06.034. Epub 2007 Aug 9. Virology. 2007. PMID: 17692355 Free PMC article.
-
MHVR-independent cell-cell spread of mouse hepatitis virus infection requires neutral pH fusion.Adv Exp Med Biol. 1995;380:351-7. doi: 10.1007/978-1-4615-1899-0_57. Adv Exp Med Biol. 1995. PMID: 8830507 Review.
-
Morphological analysis of mouse hepatitis virus A59-induced pathology with regard to viral receptor expression.Histol Histopathol. 1998 Jan;13(1):181-99. doi: 10.14670/HH-13.181. Histol Histopathol. 1998. PMID: 9476648 Review.
Cited by
-
Human coronavirus OC43 infection induces chronic encephalitis leading to disabilities in BALB/C mice.Virology. 2006 Jun 5;349(2):335-46. doi: 10.1016/j.virol.2006.01.049. Epub 2006 Mar 9. Virology. 2006. PMID: 16527322 Free PMC article.
-
D614G Substitution of SARS-CoV-2 Spike Protein Increases Syncytium Formation and Virus Titer via Enhanced Furin-Mediated Spike Cleavage.mBio. 2021 Aug 31;12(4):e0058721. doi: 10.1128/mBio.00587-21. Epub 2021 Jul 27. mBio. 2021. PMID: 34311586 Free PMC article.
-
Leveraging Artificial Intelligence and Gene Expression Analysis to Identify Some Potential Bovine Coronavirus (BCoV) Receptors and Host Cell Enzymes Potentially Involved in the Viral Replication and Tissue Tropism.Int J Mol Sci. 2025 Feb 4;26(3):1328. doi: 10.3390/ijms26031328. Int J Mol Sci. 2025. PMID: 39941096 Free PMC article.
-
Cytokine Profile Analysis During Sialodacryoadenitis Virus and Mouse Hepatitis Virus JHM Strain Infection in Primary Mixed Microglia and Astrocyte Culture-Preliminary Research.Cells. 2025 Apr 25;14(9):637. doi: 10.3390/cells14090637. Cells. 2025. PMID: 40358160 Free PMC article.
-
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and glial cells: Insights and perspectives.Brain Behav Immun Health. 2020 Aug;7:100127. doi: 10.1016/j.bbih.2020.100127. Epub 2020 Aug 13. Brain Behav Immun Health. 2020. PMID: 32838339 Free PMC article. Review.
References
-
- Beauchemin, N., P. Draber, G. Dveksler, P. Gold, S. Gray-Owen, F. Grunert, S. Hammarstrom, K. V. Holmes, A. Karlsson, M. Kuroki, S. H. Lin, L. Lucka, S. M. Najjar, M. Neumaier, B. Obrink, J. E. Shively, K. M. Skubitz, C. P. Stanners, P. Thomas, J. A. Thompson, M. Virji, S. von Kleist, C. Wagener, S. Watt, and W. Zimmermann. 1999. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp. Cell Res. 252:243-249. - PubMed
-
- Dalgleish, A. G., P. C. Beverley, P. R. Clapham, D. H. Crawford, M. F. Greaves, and R. A. Weiss. 1984. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312:763-767. - PubMed
-
- Dveksler, G. S., C. W. Dieffenbach, C. B. Cardellichio, K. McCuaig, M. N. Pensiero, G. S. Jiang, N. Beauchemin, and K. V. Holmes. 1993. Several members of the mouse carcinoembryonic antigen-related glycoprotein family are functional receptors for the coronavirus mouse hepatitis virus A59. J. Virol. 67:1-8. - PMC - PubMed
-
- Dveksler, G. S., M. N. Pensiero, C. W. Dieffenbach, C. B. Cardellichio, A. A. Basile, P. E. Elia, and K. V. Holmes. 1993. Mouse hepatitis virus strain A59 and blocking antireceptor monoclonal antibody bind to the N-terminal domain of cellular receptor. Proc. Natl. Acad. Sci. USA 90:1716-1720. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

