Unusual topological arrangement of structural motifs in the baboon reovirus fusion-associated small transmembrane protein
- PMID: 15858006
- PMCID: PMC1091723
- DOI: 10.1128/JVI.79.10.6216-6226.2005
Unusual topological arrangement of structural motifs in the baboon reovirus fusion-associated small transmembrane protein
Abstract
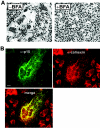
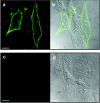
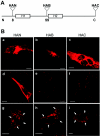
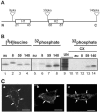
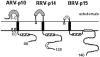
Select members of the Reoviridae are the only nonenveloped viruses known to induce syncytium formation. The fusogenic orthoreoviruses accomplish cell-cell fusion through a distinct class of membrane fusion-inducing proteins referred to as the fusion-associated small transmembrane (FAST) proteins. The p15 membrane fusion protein of baboon reovirus is unique among the FAST proteins in that it contains two hydrophobic regions (H1 and H2) recognized as potential transmembrane (TM) domains, suggesting a polytopic topology. However, detailed topological analysis of p15 indicated only the H1 domain is membrane spanning. In the absence of an N-terminal signal peptide, the H1 TM domain serves as a reverse signal-anchor to direct p15 membrane insertion and a bitopic N(exoplasmic)/C(cytoplasmic) topology. This topology results in the translocation of the smallest ectodomain ( approximately 20 residues) of any known viral fusion protein, with the majority of p15 positioned on the cytosolic side of the membrane. Mutagenic analysis indicated the unusual presence of an N-terminal myristic acid on the small p15 ectodomain is essential to the fusion process. Furthermore, the only other hydrophobic region (H2) present in p15, aside from the TM domain, is located within the endodomain. Consequently, the p15 ectodomain is devoid of a fusion peptide motif, a hallmark feature of membrane fusion proteins. The exceedingly small, myristoylated ectodomain and the unusual topological distribution of structural motifs in this nonenveloped virus membrane fusion protein necessitate alternate models of protein-mediated membrane fusion.
Figures

Similar articles
-
Cell-cell fusion induced by the avian reovirus membrane fusion protein is regulated by protein degradation.J Virol. 2004 Jun;78(11):5996-6004. doi: 10.1128/JVI.78.11.5996-6004.2004. J Virol. 2004. PMID: 15140997 Free PMC article.
-
Extensive sequence divergence and phylogenetic relationships between the fusogenic and nonfusogenic orthoreoviruses: a species proposal.Virology. 1999 Aug 1;260(2):316-28. doi: 10.1006/viro.1999.9832. Virology. 1999. PMID: 10417266
-
The NS16 protein of aquareovirus-C is a fusion-associated small transmembrane (FAST) protein, and its activity can be enhanced by the nonstructural protein NS26.Virus Res. 2013 Jan;171(1):129-37. doi: 10.1016/j.virusres.2012.11.011. Epub 2012 Nov 29. Virus Res. 2013. PMID: 23201583
-
Reovirus FAST proteins: virus-encoded cellular fusogens.Trends Microbiol. 2014 Dec;22(12):715-24. doi: 10.1016/j.tim.2014.08.005. Epub 2014 Sep 19. Trends Microbiol. 2014. PMID: 25245455 Review.
-
The reovirus fusion-associated small transmembrane (FAST) proteins: virus-encoded cellular fusogens.Curr Top Membr. 2011;68:107-40. doi: 10.1016/B978-0-12-385891-7.00005-2. Curr Top Membr. 2011. PMID: 21771497 Review. No abstract available.
Cited by
-
A virus-encoded cell-cell fusion machine dependent on surrogate adhesins.PLoS Pathog. 2008 Mar 7;4(3):e1000016. doi: 10.1371/journal.ppat.1000016. PLoS Pathog. 2008. PMID: 18369467 Free PMC article.
-
Features of a spatially constrained cystine loop in the p10 FAST protein ectodomain define a new class of viral fusion peptides.J Biol Chem. 2010 May 28;285(22):16424-33. doi: 10.1074/jbc.M110.118232. Epub 2010 Apr 2. J Biol Chem. 2010. PMID: 20363742 Free PMC article.
-
Helix-destabilizing, beta-branched, and polar residues in the baboon reovirus p15 transmembrane domain influence the modularity of FAST proteins.J Virol. 2011 May;85(10):4707-19. doi: 10.1128/JVI.02223-10. Epub 2011 Mar 2. J Virol. 2011. PMID: 21367887 Free PMC article.
-
Polycistronic Genome Segment Evolution and Gain and Loss of FAST Protein Function during Fusogenic Orthoreovirus Speciation.Viruses. 2020 Jun 29;12(7):702. doi: 10.3390/v12070702. Viruses. 2020. PMID: 32610593 Free PMC article.
-
Nonstructural protein NS17 of grass carp reovirus Honghu strain promotes virus infection by mediating cell-cell fusion and apoptosis.Virus Res. 2023 Sep;334:199150. doi: 10.1016/j.virusres.2023.199150. Epub 2023 Jun 16. Virus Res. 2023. PMID: 37302658 Free PMC article.
References
-
- Ames, J. B., R. Ishima, T. Tanaka, J. I. Gordon, L. Stryer, and M. Ikura. 1997. Molecular mechanics of calcium-myristoyl switches. Nature 389:198-202. - PubMed
-
- Beltzer, J. P., K. Fielder, C. Fuhrer, I. Geffen, C. Handschin, H. P. Wessels, and M. Spiess. 1991. Charged residues are major determinants of the transmembrane orientation of a signal-anchor sequence. J. Biol. Chem. 266:973-978. - PubMed
-
- Blumenthal, R., M. J. Clague, S. R. Durell, and R. M. Epand. 2003. Membrane fusion. Chem. Rev. 103:53-69. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

