Stimulation of poliovirus synthesis in a HeLa cell-free in vitro translation-RNA replication system by viral protein 3CDpro
- PMID: 15858019
- PMCID: PMC1091690
- DOI: 10.1128/JVI.79.10.6358-6367.2005
Stimulation of poliovirus synthesis in a HeLa cell-free in vitro translation-RNA replication system by viral protein 3CDpro
Abstract
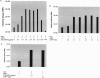
The plus-strand RNA genome of poliovirus serves three distinct functions in the life cycle of the virus. The RNA is translated and then replicated, and finally the progeny RNAs are encapsidated. These processes can be faithfully reproduced in a HeLa cell-free in vitro translation-RNA replication system that produces viable poliovirus. We have previously observed a stimulation of virus synthesis when an mRNA, encoding protein 3CD(pro), is added to the translation-RNA replication reactions of poliovirus RNA. Our aim in these experiments was to further define the factors that affect the stimulatory activity of 3CD(pro) in virus synthesis. We observed that purified 3CD(pro) protein also enhances virus synthesis by about 100-fold but has no effect on the translation of the polyprotein. Optimal stimulation is observed only when 3CD(pro) is present early in the incubation period. The stimulation, however, is abolished by a mutation either in the RNA binding domain of 3CD(pro), 3C(pro)R84S/I86A, or by each of two groups of complementary mutations R455A/R456A and D339A/S341A/D349A at interface I in the 3D(pol) domain of 3CD(pro). Surprisingly, virus synthesis is strongly inhibited by the addition of both 3C(pro) and 3CD(pro) at the beginning of incubation. We also examined the effect of other viral or cellular proteins on virus synthesis in the in vitro system. No enhancement of virus synthesis occurred with viral proteins 3BC, 3ABC, 3BCD, 3D(pol), and 3C(pro) or with cellular protein PCBP2. These results suggest that 3CD(pro) has to be present in the reaction at the time the replication complexes are assembled and that both the 3C(pro) and 3D(pol) domains of the protein are required for its activity that stimulates virus production.
Figures




Similar articles
-
Stimulation of poliovirus RNA synthesis and virus maturation in a HeLa cell-free in vitro translation-RNA replication system by viral protein 3CDpro.Virol J. 2005 Nov 21;2:86. doi: 10.1186/1743-422X-2-86. Virol J. 2005. PMID: 16300678 Free PMC article.
-
Cellular protein modification by poliovirus: the two faces of poly(rC)-binding protein.J Virol. 2007 Sep;81(17):8919-32. doi: 10.1128/JVI.01013-07. Epub 2007 Jun 20. J Virol. 2007. PMID: 17581994 Free PMC article.
-
A poliovirus 2A(pro) mutant unable to cleave 3CD shows inefficient viral protein synthesis and transactivation defects.J Virol. 1995 Oct;69(10):6280-8. doi: 10.1128/JVI.69.10.6280-6288.1995. J Virol. 1995. PMID: 7666528 Free PMC article.
-
Molecular biology and cell-free synthesis of poliovirus.Biologicals. 1993 Dec;21(4):349-56. doi: 10.1006/biol.1993.1095. Biologicals. 1993. PMID: 8024750 Review.
-
IRES-controlled protein synthesis and genome replication of poliovirus.Arch Virol Suppl. 1994;9:279-89. doi: 10.1007/978-3-7091-9326-6_28. Arch Virol Suppl. 1994. PMID: 8032259 Review.
Cited by
-
Stimulation of poliovirus RNA synthesis and virus maturation in a HeLa cell-free in vitro translation-RNA replication system by viral protein 3CDpro.Virol J. 2005 Nov 21;2:86. doi: 10.1186/1743-422X-2-86. Virol J. 2005. PMID: 16300678 Free PMC article.
-
Picornavirus morphogenesis.Microbiol Mol Biol Rev. 2014 Sep;78(3):418-37. doi: 10.1128/MMBR.00012-14. Microbiol Mol Biol Rev. 2014. PMID: 25184560 Free PMC article. Review.
-
Roles of the Picornaviral 3C Proteinase in the Viral Life Cycle and Host Cells.Viruses. 2016 Mar 17;8(3):82. doi: 10.3390/v8030082. Viruses. 2016. PMID: 26999188 Free PMC article. Review.
-
Multiple poliovirus-induced organelles suggested by comparison of spatiotemporal dynamics of membranous structures and phosphoinositides.PLoS Pathog. 2018 Apr 27;14(4):e1007036. doi: 10.1371/journal.ppat.1007036. eCollection 2018 Apr. PLoS Pathog. 2018. PMID: 29702686 Free PMC article.
-
Encapsidation of Viral RNA in Picornavirales: Studies on Cowpea Mosaic Virus Demonstrate Dependence on Viral Replication.J Virol. 2019 Jan 4;93(2):e01520-18. doi: 10.1128/JVI.01520-18. Print 2019 Jan 15. J Virol. 2019. PMID: 30355698 Free PMC article.
References
-
- Andino, R., G. E. Rieckhof, and D. Baltimore. 1990. A functional ribonucleoprotein complex forms around the 5′ end of poliovirus RNA. Cell 63:369-380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

