Endogenous hydrogen peroxide regulates the excitability of midbrain dopamine neurons via ATP-sensitive potassium channels
- PMID: 15858048
- PMCID: PMC6725114
- DOI: 10.1523/JNEUROSCI.4701-04.2005
Endogenous hydrogen peroxide regulates the excitability of midbrain dopamine neurons via ATP-sensitive potassium channels
Abstract
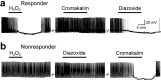
ATP-sensitive K+ (K(ATP)) channels link metabolic state to cell excitability. Here, we examined regulation of K(ATP) channels in substantia nigra dopamine neurons by hydrogen peroxide (H2O2), which is produced in all cells during aerobic metabolism. Blockade of K(ATP) channels by glibenclamide (100 nM) or depletion of intracellular H2O2 by including catalase, a peroxidase enzyme, in the patch pipette increased the spontaneous firing rate of all dopamine neurons tested in guinea pig midbrain slices. Using fluorescence imaging with dichlorofluorescein to visualize intracellular H2O2, we found that moderate increases in H2O2 during partial inhibition of glutathione (GSH) peroxidase by mercaptosuccinate (0.1-0.3 mM) had no effect on dopamine neuron firing rate. However, with greater GSH inhibition (1 mM mercaptosuccinate) or application of exogenous H2O2, 50% of recorded cells showed K(ATP) channel-dependent hyperpolarization. Responsive cells also hyperpolarized with diazoxide, a selective opener for K(ATP) channels containing sulfonylurea receptor SUR1 subunits, but not with cromakalim, a selective opener for SUR2-based channels, indicating that SUR1-based K(ATP) channels conveyed enhanced sensitivity to elevated H2O2. In contrast, when endogenous H2O2 levels were increased after inhibition of catalase, the predominant peroxidase in the substantia nigra, with 3-amino-1,2,4-triazole (1 mM), all dopamine neurons responded with glibenclamide-reversible hyperpolarization. Fluorescence imaging of H2O2 indicated that catalase inhibition rapidly amplified intracellular H2O2, whereas inhibition of GSH peroxidase, a predominantly glial enzyme, caused a slower, smaller increase, especially in nonresponsive cells. Thus, endogenous H2O2 modulates neuronal activity via K(ATP) channel opening, thereby enhancing the reciprocal relationship between metabolism and excitability.
Figures







References
-
- Albin RL, Young AB, Penney JB (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366-375. - PubMed
-
- Amoroso S, Schmid-Antomarchi H, Fosset M, Lazdunski M (1990) Glucose, sulfonylureas, and neurotransmitter release: role of ATP-sensitive K+ channels. Science 247: 852-854. - PubMed
-
- Ashcroft FM, Gribble FM (1998) Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci 21: 288-294. - PubMed
-
- Ashcroft SJ, Ashcroft FM (1990) Properties and functions of ATP-sensitive K-channels. Cell Signal 2: 197-214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
