Nerve growth factor rapidly increases muscarinic tone in mouse medial septum/diagonal band of Broca
- PMID: 15858049
- PMCID: PMC6725107
- DOI: 10.1523/JNEUROSCI.4957-04.2005
Nerve growth factor rapidly increases muscarinic tone in mouse medial septum/diagonal band of Broca
Abstract
Nerve growth factor (NGF) has been implicated in maintaining and regulating normal functioning of the septohippocampal pathway. However, many aspects of its physiological actions and the underlying mechanisms await elucidation. In this study, we investigated the effect of acute NGF exposure on neurons in the mouse medial septum/diagonal band of Broca (MS/DB), focusing on the cholinergic neurons and the subpopulation of noncholinergic neurons that were identified to be putatively GABAergic. We report that MS/DB neurons in a thin slice preparation, when exposed to NGF via bath perfusion, rapidly and indiscriminately increased the rate of spontaneous firing in all MS/DB neurons. However, focal application of NGF to individual MS/DB neurons increased spontaneous firing in cholinergic, but not in the noncholinergic, subpopulation. The NGF-induced effect on cholinergic neurons was direct, requiring activation and signaling via TrkA receptors, which were immunohistochemically localized to the cholinergic neurons in the MS/DB. TrkA receptors were absent in putative GABAergic MS/DB neurons, and blockade of TrkA signaling in these and other noncholinergic neurons had no effect on their firing activity after exposure to NGF. Conversely, methyl scopolamine, blocked the increased firing activity of noncholinergic neurons during bath perfusion of NGF. We propose a cell type-specific mode of action for NGF in the MS/DB. The neurotrophin directly enhances cholinergic neuronal activity in the MS/DB through TrkA-mediated signaling, increasing acetylcholine release and, thus, muscarinic tone. This increase in muscarinic tone, in turn, results in heightened firing activity in noncholinergic MS/DB neurons.
Figures




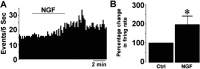

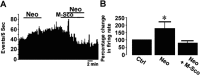

Similar articles
-
Nerve growth factor in the hippocamposeptal system: evidence for activity-dependent anterograde delivery and modulation of synaptic activity.J Neurosci. 2012 May 30;32(22):7701-10. doi: 10.1523/JNEUROSCI.0028-12.2012. J Neurosci. 2012. PMID: 22649248 Free PMC article.
-
Acute ethanol exposure elevates muscarinic tone in the septohippocampal system.J Neurophysiol. 2010 Jan;103(1):290-6. doi: 10.1152/jn.91072.2008. Epub 2009 Nov 11. J Neurophysiol. 2010. PMID: 19906873 Free PMC article.
-
Muscarinic tone sustains impulse flow in the septohippocampal GABA but not cholinergic pathway: implications for learning and memory.J Neurosci. 2000 Nov 1;20(21):8103-10. doi: 10.1523/JNEUROSCI.20-21-08103.2000. J Neurosci. 2000. PMID: 11050132 Free PMC article.
-
Nerve growth factor-independent neuronal survival: a role for NO donors.Mol Pharmacol. 2005 Oct;68(4):952-5. doi: 10.1124/mol.105.017277. Epub 2005 Jul 26. Mol Pharmacol. 2005. PMID: 16046659 Review.
-
Effects of nerve growth factor on cholinergic brain neurons.Trends Pharmacol Sci. 1989 Apr;10(4):145-9. doi: 10.1016/0165-6147(89)90166-1. Trends Pharmacol Sci. 1989. PMID: 2665246 Review.
Cited by
-
Role of vitamin d in Parkinson's disease.ISRN Neurol. 2012;2012:134289. doi: 10.5402/2012/134289. Epub 2012 Mar 7. ISRN Neurol. 2012. PMID: 22619734 Free PMC article.
-
Effects of Aging and Nerve Growth Factor on Neuropeptide Expression and Cholinergic Innervation of the Rat Basolateral Amygdala.Biology (Basel). 2024 Feb 28;13(3):155. doi: 10.3390/biology13030155. Biology (Basel). 2024. PMID: 38534426 Free PMC article.
-
Functional Diversity of Neurotrophin Actions on the Oculomotor System.Int J Mol Sci. 2016 Dec 1;17(12):2016. doi: 10.3390/ijms17122016. Int J Mol Sci. 2016. PMID: 27916956 Free PMC article. Review.
-
p75 and TrkA signaling regulates sympathetic neuronal firing patterns via differential modulation of voltage-gated currents.J Neurosci. 2009 Apr 29;29(17):5411-24. doi: 10.1523/JNEUROSCI.3503-08.2009. J Neurosci. 2009. PMID: 19403809 Free PMC article.
-
Nerve growth factor in the hippocamposeptal system: evidence for activity-dependent anterograde delivery and modulation of synaptic activity.J Neurosci. 2012 May 30;32(22):7701-10. doi: 10.1523/JNEUROSCI.0028-12.2012. J Neurosci. 2012. PMID: 22649248 Free PMC article.
References
-
- Acsády L, Pascual M, Rocamora N, Soriano E, Freund TF (2000) Nerve growth factor but not neurotrophin-3 is synthesized by hippocampal GABAergic neurons that project to the medial septum. Neuroscience 98: 23-31. - PubMed
-
- Andersen P, Bland HB, Myhrer T, Schwartzkroin PA (1979) Septohippocampal pathway necessary for dentate theta production. Brain Res 165: 13-22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
