A genetically defined mouse ovarian carcinoma model for the molecular characterization of pathway-targeted therapy and tumor resistance
- PMID: 15860581
- PMCID: PMC1087513
- DOI: 10.1073/pnas.0502256102
A genetically defined mouse ovarian carcinoma model for the molecular characterization of pathway-targeted therapy and tumor resistance
Abstract
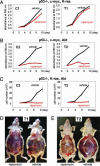
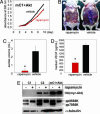
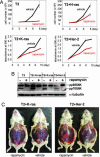
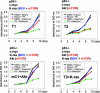
Cell lines and tumors with defined genetic alterations provide ideal systems in which to test the molecular mechanisms of tumor sensitivity to pathway-targeted therapy. We have generated mouse ovarian epithelial tumor cell lines that contain various combinations of genetic alterations in the p53, c-myc, K-ras and Akt genes. Using both in vitro and in vivo approaches, we investigated the effect of rapamycin on cell proliferation, tumor growth, and the accumulation of peritoneal ascites. We demonstrated that rapamycin effectively inhibits the growth of tumors that rely on Akt signaling for proliferation, whereas tumors in which Akt signaling is not the driving force in proliferation are resistant to rapamycin. The introduction of activated Akt to the rapamycin-resistant cells does not render the cells susceptible to rapamycin if they can use alternative pathways for survival and proliferation. Accordingly, the rapamycin-sensitive tumors develop resistance to rapamycin when presented with alternative survival pathways, such as the mitogen-activated extracellular kinase signaling pathway. The combination of rapamycin and the mitogen-activated extracellular kinase inhibitor PD98059 is required to diminish proliferation in these cell lines. Our results indicate that mammalian target of rapamycin inhibitors may be effective in a subset of tumors that depend on Akt activity for survival but not effective in all tumors that exhibit Akt activation. Tumors with alternative survival pathways may require the inactivation of multiple individual pathways for successful treatment.
Figures
Similar articles
-
Insulin-like growth factor I-mediated protection from rapamycin-induced apoptosis is independent of Ras-Erk1-Erk2 and phosphatidylinositol 3'-kinase-Akt signaling pathways.Cancer Res. 2003 Jan 15;63(2):364-74. Cancer Res. 2003. PMID: 12543789
-
RAD001 (Everolimus) delays tumor onset and progression in a transgenic mouse model of ovarian cancer.Cancer Res. 2007 Mar 15;67(6):2408-13. doi: 10.1158/0008-5472.CAN-06-4490. Cancer Res. 2007. PMID: 17363557
-
The cell survival signal Akt is differentially activated by PDGF-BB, EGF, and FGF-2 in osteoblastic cells.J Cell Biochem. 2001 Mar 26;81(2):304-11. J Cell Biochem. 2001. PMID: 11241670
-
New insights into Notch1 regulation of the PI3K-AKT-mTOR1 signaling axis: targeted therapy of γ-secretase inhibitor resistant T-cell acute lymphoblastic leukemia.Cell Signal. 2014 Jan;26(1):149-61. doi: 10.1016/j.cellsig.2013.09.021. Epub 2013 Oct 16. Cell Signal. 2014. PMID: 24140475 Review.
-
Modeling resistance to pathway-targeted therapy in ovarian cancer.Cell Cycle. 2005 Aug;4(8):1004-6. doi: 10.4161/cc.4.8.1869. Epub 2005 Aug 25. Cell Cycle. 2005. PMID: 15970711 Free PMC article. Review.
Cited by
-
Increased canonical NF-kappaB signaling specifically in macrophages is sufficient to limit tumor progression in syngeneic murine models of ovarian cancer.BMC Cancer. 2020 Oct 7;20(1):970. doi: 10.1186/s12885-020-07450-8. BMC Cancer. 2020. PMID: 33028251 Free PMC article.
-
Tumorigenesis and peritoneal colonization from fallopian tube epithelium.Oncotarget. 2015 Aug 21;6(24):20500-12. doi: 10.18632/oncotarget.3985. Oncotarget. 2015. PMID: 25971410 Free PMC article.
-
Aberrant over-expression of COX-1 intersects multiple pro-tumorigenic pathways in high-grade serous ovarian cancer.Oncotarget. 2015 Aug 28;6(25):21353-68. doi: 10.18632/oncotarget.3860. Oncotarget. 2015. PMID: 25972361 Free PMC article.
-
Targeted therapy of pyrrolo[2,3-d]pyrimidine antifolates in a syngeneic mouse model of high grade serous ovarian cancer and the impact on the tumor microenvironment.Sci Rep. 2022 Jul 5;12(1):11346. doi: 10.1038/s41598-022-14788-5. Sci Rep. 2022. PMID: 35790779 Free PMC article.
-
The mTORC1 inhibitor everolimus prevents and treats Eμ-Myc lymphoma by restoring oncogene-induced senescence.Cancer Discov. 2013 Jan;3(1):82-95. doi: 10.1158/2159-8290.CD-12-0404. Epub 2012 Dec 14. Cancer Discov. 2013. PMID: 23242809 Free PMC article.
References
-
- Holschneider, C. H. & Berek, J. S. (2000) Semin. Surg. Oncol. 19, 3–10. - PubMed
-
- Gray, J. W., Suzuki, S., Kuo, W. L., Polikoff, D., Deavers, M., Smith-McCune, K., Berchuck, A., Pinkel, D., Albertson, D. & Mills, G. B. (2003) Gynecol. Oncol. 88, S16–S21; discussion S22–S24. - PubMed
-
- Aunoble, B., Sanches, R., Didier, E. & Bignon, Y. J. (2000) Int. J. Oncol. 16, 567–576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

