Plasma chemokine levels correlate with the outcome of antiviral therapy in patients with hepatitis C
- PMID: 15860662
- PMCID: PMC1895193
- DOI: 10.1182/blood-2005-01-0126
Plasma chemokine levels correlate with the outcome of antiviral therapy in patients with hepatitis C
Abstract
Chronic infection with the hepatitis C virus (HCV) is associated with failures of T-cell-mediated immune clearance and with abnormal B-cell growth and activation. We examined the levels of chemokines that bind to CXC chemokine receptor 3 (CXCR3) to determine whether such chemokines might play a role in the failure of the immune system to clear HCV infection. Elevations in CXC ligand 9 (CXCL9), CXCL10, and CXCL11 were observed in all patients with HCV. CXCR3 expression was increased significantly on peripheral blood B lymphocytes, but not T lymphocytes, from individuals with HCV infection. Chemokine levels were measured in samples collected before, during, and after antiviral therapy from a group of 29 patients infected with HCV genotypes 1a (24 patients) and 1b (5 patients). Levels of CXCL10 and CXCL9 decreased following successful antiviral therapy; CXCL11 did not decline significantly during or in the first 6 months after therapy. The baseline level of CXCL10 (measured before the start of antiviral treatment) was greatest in patients with HCV who subsequently became nonresponders to therapy. These results suggest that plasma concentrations of immunoreactive CXCL10 may be a predictor of responsiveness or nonresponsiveness to antiviral therapy with pegylated interferon (IFN) with or without ribavirin. This observation has implications for understanding the pathogenesis of HCV infection.
Figures
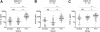

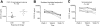
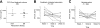

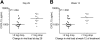
References
-
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345: 41-52. - PubMed
-
- Poynard T, Yuen MF, Ratziu V, Lai CL. Viral hepatitis C. Lancet. 2003;362: 2095-2100. - PubMed
-
- McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339: 1485-1492. - PubMed
-
- Blatt LM, Mutchnick MG, Tong MJ, et al. Assessment of hepatitis C virus RNA and genotype from 6807 patients with chronic hepatitis C in the United States. J Viral Hepat. 2000;7: 196-202. - PubMed
-
- McHutchison JG, Poynard T, Pianko S, et al. The impact of interferon plus ribavirin on response to therapy in black patients with chronic hepatitis C. The International Hepatitis Interventional Therapy Group. Gastroenterology. 2000;119: 1317-1323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

