The Shwachman-Diamond SBDS protein localizes to the nucleolus
- PMID: 15860664
- PMCID: PMC1895203
- DOI: 10.1182/blood-2005-02-0807
The Shwachman-Diamond SBDS protein localizes to the nucleolus
Abstract
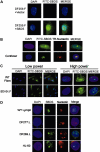
Shwachman-Diamond syndrome (SDS) is an autosomal recessively inherited disorder characterized by exocrine pancreatic insufficiency and bone marrow failure. The gene for this syndrome, SBDS, encodes a highly conserved novel protein. We characterized Shwachman-Bodian-Diamond syndrome (SBDS) protein expression and intracellular localization in 7 patients with SDS and healthy controls. As predicted by gene mutation, 4 patients with SDS exhibited no detectable full-length SBDS protein. Patient DF277, who was homozygous for the IVS2 + 2 T>C splice donor mutation, expressed scant levels of SBDS protein. Patient SD101 expressed low levels of SBDS protein harboring an R169C missense mutation. Patient DF269, who carried no detectable gene mutations, expressed wild-type levels of SBDS protein to add further support to the growing body of evidence for additional gene(s) that might contribute to the pathogenesis of the disease phenotype. The SBDS protein was detected in both the nucleus and the cytoplasm of normal control fibroblasts, but was particularly concentrated within the nucleolus. SBDS localization was cell-cycle dependent, with nucleolar localization during G1 and G2 and diffuse nuclear localization during S phase. SBDS nucleolar localization was intact in SD101 and DF269. The intranucleolar localization of SBDS provides further supportive evidence for its postulated role in rRNA processing.
Figures


References
-
- Shwachman H, Diamond LK, Oski FA, Khaw KT. The syndrome of pancreatic insufficiency and bone marrow dysfunction. J Pediatr. 1964;65: 645-663. - PubMed
-
- Bodian M, Sheldon W, Lightwood R. Congenital hypoplasia of the exocrine pancreas. Acta Paediatr. 1964;53: 282-293. - PubMed
-
- Dror Y, Freedman MH. Shwachman-diamond syndrome. Br J Haematol. 2002;118: 701-713. - PubMed
-
- Smith OP. Shwachman-Diamond syndrome. Semin Hematol. 2002;39: 95-102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

