Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells
- PMID: 15860669
- PMCID: PMC1895271
- DOI: 10.1182/blood-2004-10-4166
Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells
Abstract
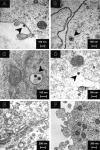
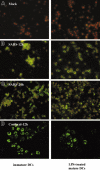
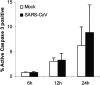
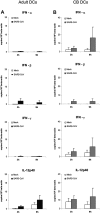
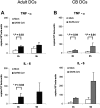
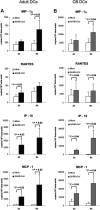
Lymphopenia and increasing viral load in the first 10 days of severe acute respiratory syndrome (SARS) suggested immune evasion by SARS-coronavirus (CoV). In this study, we focused on dendritic cells (DCs) which play important roles in linking the innate and adaptive immunity. SARS-CoV was shown to infect both immature and mature human monocyte-derived DCs by electron microscopy and immunofluorescence. The detection of negative strands of SARS-CoV RNA in DCs suggested viral replication. However, no increase in viral RNA was observed. Using cytopathic assays, no increase in virus titer was detected in infected DCs and cell-culture supernatant, confirming that virus replication was incomplete. No induction of apoptosis or maturation was detected in SARS-CoV-infected DCs. The SARS-CoV-infected DCs showed low expression of antiviral cytokines (interferon alpha [IFN-alpha], IFN-beta, IFN-gamma, and interleukin 12p40 [IL-12p40]), moderate up-regulation of proinflammatory cytokines (tumor necrosis factor alpha [TNF-alpha] and IL-6) but significant up-regulation of inflammatory chemokines (macrophage inflammatory protein 1alpha [MIP-1alpha], regulated on activation normal T cell expressed and secreted [RANTES]), interferon-inducible protein of 10 kDa [IP-10], and monocyte chemoattractant protein 1 [MCP-1]). The lack of antiviral cytokine response against a background of intense chemokine up-regulation could represent a mechanism of immune evasion by SARS-CoV.
Figures
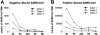

References
-
- Lai, MMC, Holmes KV. Coronaviridae: the viruses and their replication. In: Knipe DM, Howley PM, eds. Fields' virology. Philadelphia, PA: Lippincott Williams & Wilkins; 2001: 1163-1185.
-
- Choi KW, Chau TN, Tsang O, et al. Outcomes and prognostic factors in 267 patients with severe acute respiratory syndrome in Hong Kong. Ann Intern Med. 2003;139: 715-723. - PubMed
-
- Drosten C, Gunther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003; 348: 1967-1976. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

