Association of Csk to VE-cadherin and inhibition of cell proliferation
- PMID: 15861137
- PMCID: PMC1142580
- DOI: 10.1038/sj.emboj.7600647
Association of Csk to VE-cadherin and inhibition of cell proliferation
Abstract
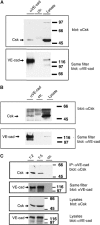
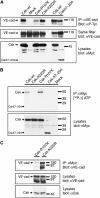
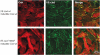
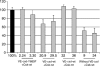
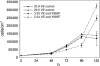
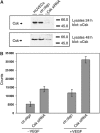
Vascular endothelial cadherin (VE-cadherin) mediates contact inhibition of cell growth in quiescent endothelial cell layers. Searching for proteins that could be involved in VE-cadherin signaling, we found the cytosolic C-terminal Src kinase (Csk), a negative regulator of Src family kinases. We show that Csk binds via its SH2 domain to the phosphorylated tyrosine 685 of VE-cadherin. VE-cadherin recruits Csk to cell contacts and both proteins can be co-precipitated from cell lysates of transfected cells and endothelial cells. Association of VE-cadherin and Csk in endothelial cells increased with increasing cell density. CHO cells expressing the tyrosine replacement mutant VE-cadherin-Y685F grow to higher cell densities than cells expressing wild-type VE-cadherin. Overexpression of Csk in these cells under an inducible promoter inhibits cell proliferation in the presence and absence of VE-cadherin, but not in the presence of VE-cadherin-Y685F. Reduction of Csk expression by RNA interference enhances endothelial cell proliferation. Our results suggest that the phosphorylated tyrosine residue 685 of VE-cadherin and probably the binding of Csk to this site are involved in inhibition of cell growth triggered by cell density.
Figures



References
-
- Breier G, Breviario F, Caveda L, Berthier R, Schnurch H, Gotsch U, Vestweber D, Risau W, Dejana E (1996) Molecular cloning and expression of murine vascular endothelial-cadherin in early stage development of cardiovascular system. Blood 87: 630–641 - PubMed
-
- Cao MY, Huber M, Beauchemin N, Famiglietti J, Albelda SM, Veillette A (1998) Regulation of mouse PECAM-1 tyrosine phosphorylation by the Src and Csk families of protein-tyrosine kinases. J Biol Chem 273: 15765–15772 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

