Quantitative analysis of adhesion and biofilm formation on hydrophilic and hydrophobic surfaces of clinical isolates of Staphylococcus epidermidis
- PMID: 15862449
- PMCID: PMC1356821
- DOI: 10.1016/j.resmic.2005.01.007
Quantitative analysis of adhesion and biofilm formation on hydrophilic and hydrophobic surfaces of clinical isolates of Staphylococcus epidermidis
Abstract
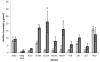
Staphylococcus epidermidis is now well established as a major nosocomial pathogen associated with infections of indwelling medical devices. The major virulence factor of these organisms is their ability to adhere to devices and form biofilms. However, it has not been established that adherence and biofilm formation are closely linked phenotypes for clinical isolates. In this study, the initial adhesion to different materials (acrylic and glass) of 9 clinical isolates of S. epidermidis, along with biofilm-positive and biofilm-negative control strains, was assayed using physico-chemical interactions to analyze the basis for bacterial adherence to the substratum. X-ray photo electron spectroscopy (XPS) analysis of the cell surface elemental composition was also performed in an attempt to find a relationship between chemical composition and adhesion capabilities. Biofilm formation on the two surfaces was evaluated by dry weight measurements. Human erythrocytes were used to evaluate the ability of S. epidermidis strains to cause hemagglutination, an indicator of the production of a poly-N-acetyl glucosamine cell surface polysaccharide also involved in biofilm formation. The clinical isolates exhibited different cell wall physico-chemical properties, resulting in differing abilities to adhere to surfaces. Adhesion to hydrophobic substrata for all strains occurred to a greater extent than that to hydrophilic surfaces. Bacterial cell hydrophobicity seemed to have little or no influence on adhesion. X-ray photoelectron spectroscopy analysis showed a high ratio of oxygen/carbon for all strains, which is a common characteristic of S. epidermidis species. No relevant relationship was found between XPS data and adhesion values. All strains forming biofilms were able to agglutinate erythrocytes. However, no direct relationship was found between the amount of biofilm formed and the initial adhesion extent. These results indicate that high levels of initial adherence do not necessarily lead to thick biofilm formation. These two aspects of the pathogenesis of medical device related-infection may need to be evaluated independently to ascertain the contribution of each to the virulence of S. epidermidis causing device-related infections.
Figures
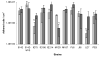
References
-
- An Y, Friedman R. Laboratory methods for studies of bacterial adhesion. J Microbiol Meth. 1997;30:141–152.
-
- Azeredo J, Oliveira R. The role of exopolymers in the attachment of Sphingomonas paucimobilis. Biofouling. 2000;61:59–67.
-
- J. Azeredo, R. Oliveira, in: P. Lens, A.P. Moran, T. Mahoney, P. Stoodley, V. O’Flaherty (Eds.), Biofilms in Medicine, Industry and Environmental Biotechnology—Characteristics, Analysis and Control, IWA Publishing, London, 2003, pp. 16–31.
-
- Bos R, van der Mei H, Busscher H. Physico-chemistry of initial microbial adhesive interactions—its mechanisms and methods for study. FEMS Microbiol Rev. 1999;23:179–230. - PubMed
-
- Briandet R, Herry J, Bellon-Fontaine M. Determination of the van der Waals, electron donor and electron acceptor surface tension components of static Gram-positive microbial biofilms. Colloids Surf B Biointerfaces. 2001;21:299–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

