Effects of probiotics on atopic dermatitis: a randomised controlled trial
- PMID: 15863468
- PMCID: PMC1720555
- DOI: 10.1136/adc.2004.060673
Effects of probiotics on atopic dermatitis: a randomised controlled trial
Abstract
Background: The aim of the study was to investigate the effects of probiotics on moderate or severe atopic dermatitis (AD) in young children.
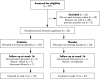
Methods: Fifty six children aged 6-18 months with moderate or severe AD were recruited into a randomised double blind placebo controlled trial in Perth, Western Australia; 53 children completed the study. The children were given a probiotic (1x10(9)Lactobacillus fermentum VRI-033 PCC; Probiomics) or an equivalent volume of placebo, twice daily for 8 weeks. A final assessment at 16 weeks was performed.
Results: The main outcome measures were severity and extent of AD at the end of the study, as measured by the Severity Scoring of Atopic Dermatitis (SCORAD) index. The reduction in the SCORAD index over time was significant in the probiotic group (p = 0.03) but not the placebo group. Significantly more children receiving probiotics (n = 24, 92%) had a SCORAD index that was better than baseline at week 16 compared with the placebo group (n = 17, 63%) (p = 0.01). At the completion of the study more children in the probiotic group had mild AD (n = 14, 54%) compared to the placebo group (n = 8, 30%).
Conclusion: Supplementation with probiotic L fermentum VRI-003 PCC is beneficial in improving the extent and severity of AD in young children with moderate or severe disease.
Figures

Comment in
-
Probiotics as mainstream allergy therapy?Arch Dis Child. 2005 Sep;90(9):881-2. doi: 10.1136/adc.2005.073114. Arch Dis Child. 2005. PMID: 16113122 Free PMC article. No abstract available.
-
Probiotics help reduce severity of atopic dermatitis.J Pediatr. 2006 Jan;148(1):143-4. doi: 10.1016/j.jpeds.2005.12.006. J Pediatr. 2006. PMID: 16440481 No abstract available.
-
Effects of probiotics on atopic dermatitis? Additional studies are still needed!Arch Dis Child. 2006 Mar;91(3):276; author reply 276. Arch Dis Child. 2006. PMID: 16492896 Free PMC article. No abstract available.
-
Effects of probiotics on atopic dermatitis.Arch Dis Child. 2006 Apr;91(4):373. Arch Dis Child. 2006. PMID: 16551799 Free PMC article. No abstract available.
-
Two "positive" studies of probiotics for atopic dermatitis: or are they?Arch Dermatol. 2006 Sep;142(9):1201-3. doi: 10.1001/archderm.142.9.1201. Arch Dermatol. 2006. PMID: 16983007 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
