The fast gating mechanism in ClC-0 channels
- PMID: 15863476
- PMCID: PMC1366516
- DOI: 10.1529/biophysj.104.053447
The fast gating mechanism in ClC-0 channels
Abstract
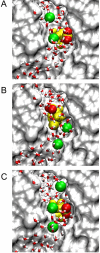
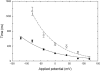
We investigate and then modify the hypothesis that a glutamate side chain acts as the fast gate in ClC-0 channels. We first create a putative open-state configuration of the prokaryotic ClC Cl- channel using its crystallographic structure as a basis. Then, retaining the same pore shape, the prokaryotic ClC channel is converted to ClC-0 by replacing all the nonconserved polar and charged residues. Using this open-state channel model, we carry out molecular dynamics simulations to study how the glutamate side chain can move between open and closed configurations. When the side chain extends toward the extracellular end of the channel, it presents an electrostatic barrier to Cl- conduction. However, external Cl- ions can push the side chain into a more central position where, pressed against the channel wall, it does not impede the motion of Cl- ions. Additionally, a proton from a low-pH external solution can neutralize the extended glutamate side chain, which also removes the barrier to conduction. Finally, we use Brownian dynamics simulations to demonstrate the influence of membrane potential and external Cl- concentration on channel open probability.
Figures




Similar articles
-
Exterior site occupancy infers chloride-induced proton gating in a prokaryotic homolog of the ClC chloride channel.Biophys J. 2004 Sep;87(3):1686-96. doi: 10.1529/biophysj.104.042465. Biophys J. 2004. PMID: 15345547 Free PMC article.
-
Conduction mechanisms of chloride ions in ClC-type channels.Biophys J. 2004 Feb;86(2):846-60. doi: 10.1016/S0006-3495(04)74160-0. Biophys J. 2004. PMID: 14747320 Free PMC article.
-
An energy-efficient gating mechanism in the acetylcholine receptor channel suggested by molecular and Brownian dynamics.Biophys J. 2006 Feb 1;90(3):799-810. doi: 10.1529/biophysj.105.067868. Epub 2005 Nov 11. Biophys J. 2006. PMID: 16284265 Free PMC article.
-
Structural insights into chloride and proton-mediated gating of CLC chloride channels.Biochemistry. 2004 Feb 10;43(5):1135-44. doi: 10.1021/bi0359776. Biochemistry. 2004. PMID: 14756549 Review.
-
Coupling gating with ion permeation in ClC channels.Sci STKE. 2003 Jun 24;2003(188):pe23. doi: 10.1126/stke.2003.188.pe23. Sci STKE. 2003. PMID: 12824475 Review.
Cited by
-
Modeling and simulation of ion channels.Chem Rev. 2012 Dec 12;112(12):6250-84. doi: 10.1021/cr3002609. Epub 2012 Oct 4. Chem Rev. 2012. PMID: 23035940 Free PMC article. Review. No abstract available.
-
A tale of two CLCs: biophysical insights toward understanding ClC-5 and ClC-7 function in endosomes and lysosomes.J Physiol. 2015 Sep 15;593(18):4139-50. doi: 10.1113/JP270604. Epub 2015 Jun 26. J Physiol. 2015. PMID: 26036722 Free PMC article. Review.
-
Influences of mutations on the electrostatic binding free energies of chloride ions in Escherichia coli ClC.J Phys Chem B. 2012 Jun 7;116(22):6431-8. doi: 10.1021/jp300430f. Epub 2012 May 29. J Phys Chem B. 2012. PMID: 22612693 Free PMC article.
-
A three-state multi-ion kinetic model for conduction properties of ClC-0 chloride channel.Biophys J. 2010 Jul 21;99(2):464-71. doi: 10.1016/j.bpj.2010.04.047. Biophys J. 2010. PMID: 20643064 Free PMC article.
-
The mechanism of fast-gate opening in ClC-0.J Gen Physiol. 2007 Oct;130(4):335-49. doi: 10.1085/jgp.200709759. Epub 2007 Sep 10. J Gen Physiol. 2007. PMID: 17846164 Free PMC article.
References
-
- Jentsch, T. J., T. Friedrich, A. Schriever, and H. Yamada. 1999. The ClC chloride channel family. Pflugers Arch. 437:783–795. - PubMed
-
- Maduke, M., C. Miller, and J. A. Mindell. 2000. A decade of ClC chloride channels: structure, mechanism, and many unsettled questions. Annu. Rev. Biophys. Biomol. Struct. 29:411–438. - PubMed
-
- Fahlke, C. 2001. Ion permeation and selectivity in ClC-type chloride channels. Am. J. Physiol. Renal Physiol. 280:F748–F757. - PubMed
-
- Jentsch, T. J., V. Stein, F. Weinreich, and A. A. Zdebik. 2002. Molecular structure and physiological function of chloride channels. Physiol. Rev. 82:503–568. - PubMed
-
- Miller, C. 1982. Open-state substructure of single chloride channels from Torpedo electroplax. Phil. Trans. Roy. Soc. Lond. B. Biol. Sci. B299:401–411. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

