Destination-selective long-distance movement of phloem proteins
- PMID: 15863519
- PMCID: PMC1143078
- DOI: 10.1105/tpc.105.031419
Destination-selective long-distance movement of phloem proteins
Abstract
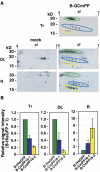
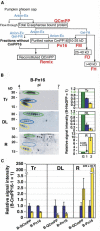
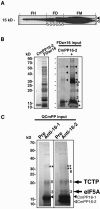
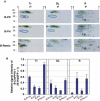
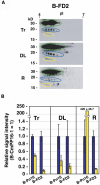
The phloem macromolecular transport system plays a pivotal role in plant growth and development. However, little information is available regarding whether the long-distance trafficking of macromolecules is a controlled process or passive movement. Here, we demonstrate the destination-selective long-distance trafficking of phloem proteins. Direct introduction, into rice (Oryza sativa), of phloem proteins from pumpkin (Cucurbita maxima) was used to screen for the capacity of specific proteins to move long distance in rice sieve tubes. In our system, shoot-ward translocation appeared to be passively carried by bulk flow. By contrast, root-ward movement of the phloem RNA binding proteins 16-kD C. maxima phloem protein 1 (CmPP16-1) and CmPP16-2 was selectively controlled. When CmPP16 proteins were purified, the root-ward movement of CmPP16-1 became inefficient, suggesting the presence of pumpkin phloem factors that are responsible for determining protein destination. Gel-filtration chromatography and immunoprecipitation showed that CmPP16-1 formed a complex with other phloem sap proteins. These interacting proteins positively regulated the root-ward movement of CmPP16-1. The same proteins interacted with CmPP16-2 as well and did not positively regulate its root-ward movement. Our data demonstrate that, in addition to passive bulk flow transport, a destination-selective process is involved in long-distance movement control, and the selective movement is regulated by protein-protein interaction in the phloem sap.
Figures



References
-
- Arnon, D.I., and Hoagland, D.R. (1940). Crop production in artificial solutions and soils with special reference to factors influencing yield and absorption of inorganic nutrients. Soil Sci. 50, 463–471.
-
- Barnes, A., Bale, J., Constantinidou, C., Ashton, P., Jones, A., and Pritchard, J. (2004). Determining protein identity from sieve element sap in Ricinus communis L. by quadrupole time of flight (Q-TOF) mass spectrometry. J. Exp. Bot. 55, 1473–1481. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

