Caspase inhibition reduces tubular apoptosis and proliferation and slows disease progression in polycystic kidney disease
- PMID: 15863619
- PMCID: PMC1100753
- DOI: 10.1073/pnas.0408518102
Caspase inhibition reduces tubular apoptosis and proliferation and slows disease progression in polycystic kidney disease
Abstract
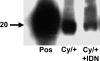
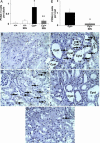
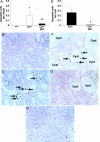
We have previously demonstrated an increase in proapoptotic caspase-3 in the kidney of Han:SPRD rats with polycystic kidney disease (PKD). The aim of the present study was to determine the effect of caspase inhibition on tubular cell apoptosis and proliferation, cyst formation, and renal failure in the Han:SPRD rat model of PKD. Heterozygous (Cy/+) and littermate control (+/+) male rats were weaned at 3 weeks of age and then treated with the caspase inhibitor IDN-8050 (10 mg/kg per day) by means of an Alzet (Palo Alto, CA) minipump or vehicle [polyethylene glycol (PEG 300)] for 5 weeks. The two-kidney/total body weight ratio more than doubled in Cy/+ rats compared with +/+ rats. IDN-8050 significantly reduced the kidney enlargement by 44% and the cyst volume density by 29% in Cy/+ rats. Cy/+ rats with PKD have kidney failure as indicated by a significant increase in blood urea nitrogen. IDN-8050 significantly reduced the increase in blood urea nitrogen in the Cy/+ rats. The number of proliferating cell nuclear antigen-positive tubular cells and apoptotic tubular cells in non-cystic and cystic tubules was significantly reduced in IDN-8050-treated Cy/+ rats compared with vehicle-treated Cy/+ rats. On immunoblot, the active form of caspase-3 (20 kDa) was significantly decreased in IDN-8050-treated Cy/+ rats compared with vehicle-treated Cy/+ rats. In summary, in a rat model of PKD, caspase inhibition with IDN-8050 (i) decreases apoptosis and proliferation in cystic and noncystic tubules; (ii) inhibits renal enlargement and cystogenesis, and (iii) attenuates the loss of kidney function.
Figures
Similar articles
-
Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease.J Am Soc Nephrol. 2005 Jan;16(1):46-51. doi: 10.1681/ASN.2004080660. Epub 2004 Nov 24. J Am Soc Nephrol. 2005. PMID: 15563559
-
An mTOR kinase inhibitor slows disease progression in a rat model of polycystic kidney disease.Nephrol Dial Transplant. 2015 Jan;30(1):45-53. doi: 10.1093/ndt/gfu296. Epub 2014 Sep 19. Nephrol Dial Transplant. 2015. PMID: 25239638 Free PMC article.
-
Caspases, Bcl-2 proteins and apoptosis in autosomal-dominant polycystic kidney disease.Kidney Int. 2002 Apr;61(4):1220-30. doi: 10.1046/j.1523-1755.2002.00250.x. Kidney Int. 2002. PMID: 11918728
-
What is the role of tubular epithelial cell apoptosis in polycystic kidney disease (PKD)?Cell Cycle. 2005 Nov;4(11):1550-4. doi: 10.4161/cc.4.11.2185. Epub 2005 Nov 17. Cell Cycle. 2005. PMID: 16258272 Review.
-
Apoptosis and autophagy in polycystic kidney disease (PKD).Cell Signal. 2020 Apr;68:109518. doi: 10.1016/j.cellsig.2019.109518. Epub 2019 Dec 24. Cell Signal. 2020. PMID: 31881325 Free PMC article. Review.
Cited by
-
Loss of GM3 synthase gene, but not sphingosine kinase 1, is protective against murine nephronophthisis-related polycystic kidney disease.Hum Mol Genet. 2012 Aug 1;21(15):3397-407. doi: 10.1093/hmg/dds172. Epub 2012 May 4. Hum Mol Genet. 2012. PMID: 22563011 Free PMC article.
-
Molecular Mechanisms of Epigenetic Regulation, Inflammation, and Cell Death in ADPKD.Front Mol Biosci. 2022 Jun 29;9:922428. doi: 10.3389/fmolb.2022.922428. eCollection 2022. Front Mol Biosci. 2022. PMID: 35847973 Free PMC article. Review.
-
Urinary biomarkers for monitoring disease progression in the Han:SPRD-cy rat model of autosomal-dominant polycystic kidney disease.Comp Med. 2010 Dec;60(6):448-54. Comp Med. 2010. PMID: 21262131 Free PMC article.
-
Polycystins and cellular Ca2+ signaling.Cell Mol Life Sci. 2013 Aug;70(15):2697-712. doi: 10.1007/s00018-012-1188-x. Epub 2012 Oct 18. Cell Mol Life Sci. 2013. PMID: 23076254 Free PMC article. Review.
-
Macrophage migration inhibitory factor is regulated by HIF-1α and cAMP and promotes renal cyst cell proliferation in a macrophage-independent manner.J Mol Med (Berl). 2020 Nov;98(11):1547-1559. doi: 10.1007/s00109-020-01964-1. Epub 2020 Sep 4. J Mol Med (Berl). 2020. PMID: 32885302 Free PMC article.
References
-
- Fick-Brosnahan, G, Ecder, T. & Schrier, R. W. (2001) in Diseases of the Kidney and Urinary Tract, ed. Schrier R. W (Lippincott, Williams & Wilkins, Philadelphia), pp. 547–588.
-
- Cowley, B. D., Jr., Gudapaty, S., Kraybill, A. L., Barash, B. D., Harding, M. A., Calvet, J. P. & Gattone, V. H. 2. (1993) Kidney Int. 43, 522–534. - PubMed
-
- Kranzlin, B., Schieren, G. & Gretz, N. (1997) Kidney Int. 51, 1160–1169. - PubMed
-
- Schafer, K., Gretz, N., Bader, M., Oberbaumer, I., Eckardt, K. U., Kriz, W. & Bachmann, S. (1994) Kidney Int. 46, 134–152. - PubMed
-
- Hocher, B., Zart, R., Schwarz, A., Vogt, V., Braun, C., Thone-reineke, C., Braun, N., Neumayer, H., Koppenhagen, K., Bauer, C., et al. (1999) J. Am. Soc. Nephrol. 9, 1169–1177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

