Dissociation of heterochromatin protein 1 from lamin B receptor induced by human polyomavirus agnoprotein: role in nuclear egress of viral particles
- PMID: 15864296
- PMCID: PMC1299312
- DOI: 10.1038/sj.embor.7400406
Dissociation of heterochromatin protein 1 from lamin B receptor induced by human polyomavirus agnoprotein: role in nuclear egress of viral particles
Abstract
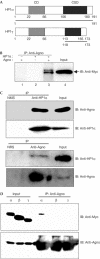
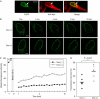
The nuclear envelope is one of the chief obstacles to the translocation of macromolecules that are larger than the diameter of nuclear pores. Heterochromatin protein 1 (HP1) bound to the lamin B receptor (LBR) is thought to contribute to reassembly of the nuclear envelope after cell division. Human polyomavirus agnoprotein (Agno) has been shown to bind to HP1alpha and to induce its dissociation from LBR, resulting in destabilization of the nuclear envelope. Fluorescence recovery after photobleaching showed that Agno increased the lateral mobility of LBR in the inner nuclear membrane. Biochemical and immunofluorescence analyses showed that Agno is targeted to the nuclear envelope and facilitates the nuclear egress of polyomavirus-like particles. These results indicate that dissociation of HP1alpha from LBR and consequent perturbation of the nuclear envelope induced by polyomavirus Agno promote the translocation of virions out of the nucleus.
Figures



Similar articles
-
[Recent research on the JC virus].No To Shinkei. 2007 Feb;59(2):101-8. No To Shinkei. 2007. PMID: 17315751 Review. Japanese.
-
[Recent research on the JC virus].Brain Nerve. 2007 Feb;59(2):101-8. Brain Nerve. 2007. PMID: 17380774 Review. Japanese.
-
Interaction between an integral protein of the nuclear envelope inner membrane and human chromodomain proteins homologous to Drosophila HP1.J Biol Chem. 1996 Jun 21;271(25):14653-6. doi: 10.1074/jbc.271.25.14653. J Biol Chem. 1996. PMID: 8663349
-
Fate of the inner nuclear membrane protein lamin B receptor and nuclear lamins in herpes simplex virus type 1 infection.J Virol. 2001 Sep;75(18):8818-30. doi: 10.1128/jvi.75.18.8818-8830.2001. J Virol. 2001. PMID: 11507226 Free PMC article.
-
Retention and mobility of the mammalian lamin B receptor in the plant nuclear envelope.Biol Cell. 2007 Oct;99(10):553-62. doi: 10.1042/bc20070033. Biol Cell. 2007. PMID: 17868028
Cited by
-
Nuclear Cytoskeleton in Virus Infection.Int J Mol Sci. 2022 Jan 5;23(1):578. doi: 10.3390/ijms23010578. Int J Mol Sci. 2022. PMID: 35009004 Free PMC article. Review.
-
Micronuclei Formation Is Prevented by Aurora B-Mediated Exclusion of HP1a from Late-Segregating Chromatin in Drosophila.Genetics. 2018 Sep;210(1):171-187. doi: 10.1534/genetics.118.301031. Epub 2018 Jul 9. Genetics. 2018. PMID: 29986897 Free PMC article.
-
The human polyoma JC virus agnoprotein acts as a viroporin.PLoS Pathog. 2010 Mar 12;6(3):e1000801. doi: 10.1371/journal.ppat.1000801. PLoS Pathog. 2010. PMID: 20300659 Free PMC article.
-
Nuclear magnetic resonance structure revealed that the human polyomavirus JC virus agnoprotein contains an α-helix encompassing the Leu/Ile/Phe-rich domain.J Virol. 2014 Jun;88(12):6556-75. doi: 10.1128/JVI.00146-14. Epub 2014 Mar 26. J Virol. 2014. PMID: 24672035 Free PMC article.
-
BKV agnoprotein interacts with α-soluble N-ethylmaleimide-sensitive fusion attachment protein, and negatively influences transport of VSVG-EGFP.PLoS One. 2011;6(9):e24489. doi: 10.1371/journal.pone.0024489. Epub 2011 Sep 12. PLoS One. 2011. PMID: 21931730 Free PMC article.
References
-
- Beaudouin J, Gerlich D, Daigle N, Eils R, Ellenberg J (2002) Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell 108: 83–96 - PubMed
-
- Gerlich D, Beaudouin J, Gebhard M, Ellenberg J, Eils R (2001) Four-dimensional imaging and quantitative reconstruction to analyse complex spatiotemporal processes in live cells. Nat Cell Biol 3: 852–855 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

