Regulation of CD1d expression and function by a herpesvirus infection
- PMID: 15864354
- PMCID: PMC1087176
- DOI: 10.1172/JCI24041
Regulation of CD1d expression and function by a herpesvirus infection
Abstract
Little is known about the role of CD1d-restricted T cells in antiviral immune responses. Here we show that the lytic replication cycle of the Kaposi sarcoma-associated herpesvirus (KSHV) promotes downregulation of cell-surface CD1d. This is caused by expression of the 2 modulator of immune recognition (MIR) proteins of the virus, each of which promotes the loss of surface CD1d expression following transfection into uninfected cells. Inhibition of CD1d surface expression is due to ubiquitination of the CD1d alpha-chain on a unique lysine residue in its cytoplasmic tail, which triggers endocytosis. Unlike MIR-mediated MHC class I downregulation, however, CD1d downregulation does not appear to include accelerated lysosomal degradation. MIR2-induced downregulation of CD1d results in reduced activation of CD1d-restricted T cells in vitro. KSHV modulation of CD1d expression represents a strategy for viral evasion of innate host immune responses and implicates CD1d-restricted T cells as regulators of this viral infection.
Figures


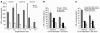


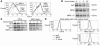
Comment in
-
A seek-and-hide game between Cd1-restricted T cells and herpesviruses.J Clin Invest. 2005 May;115(5):1146-9. doi: 10.1172/JCI25000. J Clin Invest. 2005. PMID: 15864346 Free PMC article.
References
-
- Katze MG, He Y, Gale M., Jr Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2002;2:675–687. - PubMed
-
- French AR, Yokoyama WM. Natural killer cells and viral infections. Curr. Opin. Immunol. 2003;15:45–51. - PubMed
-
- Gumperz JE, Brenner MB. CD1-specific T cells in microbial immunity. Curr. Opin. Immunol. 2001;13:471–478. - PubMed
-
- Porcelli SA, Modlin RL. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu. Rev. Immunol. 1999;17:297–329. - PubMed
-
- Beckman EM, et al. CD1c restricts responses of mycobacteria-specific T cells. Evidence for antigen presentation by a second member of the human CD1 family. J. Immunol. 1996;157:2795–2803. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

