Mechanistic studies of mouse polyamine oxidase with N1,N12-bisethylspermine as a substrate
- PMID: 15865452
- PMCID: PMC1635011
- DOI: 10.1021/bi050347k
Mechanistic studies of mouse polyamine oxidase with N1,N12-bisethylspermine as a substrate
Abstract
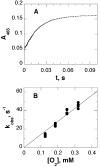
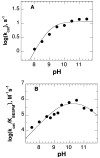
In mammalian cells, the flavoprotein polyamine oxidase catalyzes a key step in the catabolism of polyamines, the oxidation of N1-acetylspermine and N1-acetylspermidine to spermidine and putrescine, respectively. The mechanism of the mouse enzyme has been studied with N1,N12-bisethylspermine (BESPM) as a substrate. At pH 10, the pH optimum, the limiting rate of reduction of the flavin in the absence of oxygen is comparable to the k(cat) value for turnover, establishing reduction as rate-limiting. Oxidation of the reduced enzyme is a simple second-order reaction. No intermediates are seen in the reductive or oxidative half-reactions. The k(cat) value decreases below a pK(a) of 9.0. The k(cat)/K(m) value for BESPM exhibits a bell-shaped pH profile, with pK(a) values of 9.8 and 10.8. These pK(a) values are assigned to the substrate nitrogens. The rate constant for the reaction of the reduced enzyme with oxygen is not affected by a pH between 7.5 and 10. Active site residue Tyr430 is conserved in the homologous protein monoamine oxidase. Mutation of this residue to phenylalanine results in a 6-fold decrease in the k(cat) value and the k(cat)/K(m) value for oxygen due to a comparable decrease in the rate constant for flavin reduction. This moderate change is not consistent with this residue forming a tyrosyl radical during catalysis.
Figures

References
-
- Tabor CW, Tabor H. Polyamines. Annu. Rev. Biochem. 1984;53:749–790. - PubMed
-
- Marton LJ, Pegg AE. Polyamines as targets for therapeutic intervention. Annu. Rev. Pharmacol. Toxicol. 1995;35:55–91. - PubMed
-
- Bergeron RJ, Feng Y, Weimar WR, McManis JS, Dimova H, Porter C, Raisler B, Phanstiel O. A comparison of structure–activity relationships between spermidine and spermine analogue antineoplastics. J. Med. Chem. 1997;40:1475–1494. - PubMed
-
- Bitonti AJ, Dumont JA, Bush TL, Stemerick DM, Edwards ML. Bis(benzyl)polyamine analogs as novel substrates for polyamine oxidase. J. Biol. Chem. 1990;265:382–388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

