Enhanced laminin binding by alpha-dystroglycan after enzymatic deglycosylation
- PMID: 15865602
- PMCID: PMC1184583
- DOI: 10.1042/BJ20050375
Enhanced laminin binding by alpha-dystroglycan after enzymatic deglycosylation
Abstract
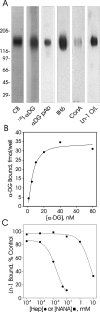
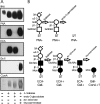
Carbohydrate modifications are clearly important to the function of alpha-dystroglycan but their composition and structure remain poorly understood. In the present study, we describe experiments aimed at identifying the alpha-dystroglycan oligosaccharides important for its binding to laminin-1 and carbohydrate-dependent mAbs (monoclonal antibodies) IIH6 and VIA4(1). We digested highly purified skeletal muscle alpha-dystroglycan with an array of linkage-specific endo- and exoglycosidases, which were verified for action on alpha-dystroglycan by loss/gain of reactivity for lectins with defined glyco-epitopes. Notably, digestion with a combination of Arthrobacter ureafaciens sialidase, beta(1-4)galactosidase and beta-N-acetylglucosaminidase substantially degraded SiaAalpha2-3Galbeta1-4GlcNAcbeta1-2Man glycans on highly purified alpha-dystroglycan that nonetheless exhibited enhanced IIH6, VIA4(1) and laminin-1 binding activity. Additional results indicate that alpha-dystroglycan is probably modified with other anionic sugars besides sialic acid and suggest that rare alpha-linked GlcNAc moieties may block its complete deglycosylation with currently available enzymes.
Figures



References
-
- Blake D. J., Weir A., Newey S. E., Davies K. E. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol. Rev. 2002;82:291–329. - PubMed
-
- Ervasti J. M. Costameres: the Achilles' heel of Herculean muscle. J. Biol. Chem. 2003;278:13591–13594. - PubMed
-
- Michele D. E., Campbell K. P. Dystrophin–glycoprotein complex: post-translational processing and dystroglycan function. J. Biol. Chem. 2003;278:15457–15460. - PubMed
-
- Cote P. D., Moukhles H., Lindenbaum M., Carbonetto S. Chimaeric mice deficient in dystroglycans develop muscular dystrophy and have disrupted myoneural synapses. Nat. Genet. 1999;23:338–342. - PubMed
-
- Cohn R. D., Henry M. D., Michele D. E., Barresi R., Saito F., Moore S. A., Flanagan J. D., Skwarchuk M. W., Robbins M. E., Mendell J. R., et al. Disruption of DAG1 in differentiated skeletal muscle reveals a role for dystroglycan in muscle regeneration. Cell (Cambridge, Mass.) 2002;110:639–648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

