Transgenic inhibition of Nogo-66 receptor function allows axonal sprouting and improved locomotion after spinal injury
- PMID: 15866044
- PMCID: PMC3246391
- DOI: 10.1016/j.mcn.2004.12.008
Transgenic inhibition of Nogo-66 receptor function allows axonal sprouting and improved locomotion after spinal injury
Abstract
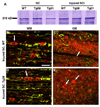
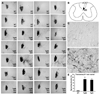
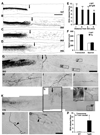
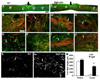
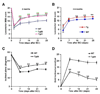
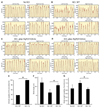
Axon growth after spinal injury is thought to be limited in part by myelin-derived proteins that act via the Nogo-66 Receptor (NgR). To test this hypothesis, we sought to study recovery from spinal cord injury (SCI) after inhibiting NgR transgenically with a soluble function-blocking NgR fragment. Glial fibrillary acidic protein (gfap) gene regulatory elements were used to generate mice that secrete NgR(310)ecto from astrocytes. After mid-thoracic dorsal over-hemisection injury, gfap::ngr(310)ecto mice exhibit enhanced raphespinal and corticospinal axonal sprouting into the lumbar spinal cord. Recovery of locomotion is improved in the gfap::ngr(310)ecto mice. These data indicate that the NgR ligands, Nogo-66, MAG, and OMgp, play a role in limiting axonal growth in the injured adult CNS and that NgR(310)ecto might provide a therapeutic means to promote recovery from SCI.
Figures



Similar articles
-
Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury.Neuron. 2004 Oct 28;44(3):439-51. doi: 10.1016/j.neuron.2004.10.015. Neuron. 2004. PMID: 15504325
-
Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury.J Neurosci. 2004 Nov 17;24(46):10511-20. doi: 10.1523/JNEUROSCI.2828-04.2004. J Neurosci. 2004. PMID: 15548666 Free PMC article.
-
The Nogo receptor, its ligands and axonal regeneration in the spinal cord; a review.J Neurocytol. 2002 Feb;31(2):93-120. doi: 10.1023/a:1023941421781. J Neurocytol. 2002. PMID: 12815233 Review.
-
Immunization with recombinant Nogo-66 receptor (NgR) promotes axonal regeneration and recovery of function after spinal cord injury in rats.Neurobiol Dis. 2008 Dec;32(3):535-42. doi: 10.1016/j.nbd.2008.09.012. Epub 2008 Sep 30. Neurobiol Dis. 2008. PMID: 18930141
-
NgR acts as an inhibitor to axonal regeneration in adults.Front Biosci. 2008 Jan 1;13:2030-40. doi: 10.2741/2821. Front Biosci. 2008. PMID: 17981689 Review.
Cited by
-
The Nogo-66 receptor NgR1 is required only for the acute growth cone-collapsing but not the chronic growth-inhibitory actions of myelin inhibitors.J Neurosci. 2007 Jul 4;27(27):7117-24. doi: 10.1523/JNEUROSCI.1541-07.2007. J Neurosci. 2007. PMID: 17611264 Free PMC article.
-
Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord.J Neurosci. 2010 Feb 3;30(5):1657-76. doi: 10.1523/JNEUROSCI.3111-09.2010. J Neurosci. 2010. PMID: 20130176 Free PMC article.
-
Perinatal asphyxia: current status and approaches towards neuroprotective strategies, with focus on sentinel proteins.Neurotox Res. 2011 May;19(4):603-27. doi: 10.1007/s12640-010-9208-9. Epub 2010 Jul 20. Neurotox Res. 2011. PMID: 20645042 Free PMC article. Review.
-
Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury.J Neurosci. 2006 Oct 18;26(42):10856-67. doi: 10.1523/JNEUROSCI.2980-06.2006. J Neurosci. 2006. PMID: 17050723 Free PMC article.
-
Memory impairment in transgenic Alzheimer mice requires cellular prion protein.J Neurosci. 2010 May 5;30(18):6367-74. doi: 10.1523/JNEUROSCI.0395-10.2010. J Neurosci. 2010. PMID: 20445063 Free PMC article.
References
-
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. - PubMed
-
- Bartsch U, Bandtlow CE, Schnell L, Bartsch S, Spillmann AA, Rubin BP, Hillenbrand R, Montag D, Schwab ME, Schachner M. Lack of evidence that myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron. 1995;15:1375–1381. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ, Nockels R, Perot PL, Salzman SK, Young W. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter animal spinal cord injury study. J. Neurotrauma. 1996;13:343–359. - PubMed
-
- Benfey M, Aguayo AJ. Extensive elongation of axons from rat brain into peripheral nerve grafts. Nature. 1982;296:150–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

