Natural and derivative brevetoxins: historical background, multiplicity, and effects
- PMID: 15866774
- PMCID: PMC1257558
- DOI: 10.1289/ehp.7499
Natural and derivative brevetoxins: historical background, multiplicity, and effects
Abstract
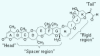
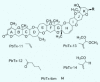
Symptoms consistent with inhalation toxicity have long been associated with Florida red tides, and various causal agents have been proposed. Research since 1981 has centered on a group of naturally occurring trans-fused cyclic polyether compounds called brevetoxins that are produced by a marine dinoflagellate known as Karenia brevis. Numerous individual brevetoxins have been identified from cultures as well as from natural bloom events. A spectrum of brevetoxin derivatives produced by chemical modification of the natural toxins has been prepared to examine the effects of functional group modification on physiologic activity. Certain structural features of natural and synthetic derivatives of brevetoxin appear to ascribe specific physiologic consequences to each toxin. Differential physiologic effects have been documented with many of the natural toxins and derivatives, reinforcing the hypothesis that metabolism or modification of toxin structures modulates both the specific toxicity (lethality on a per milligram basis) and potentially the molecular mechanism(s) of action. A series of naturally occurring fused-ring polyether compounds with fewer rings than brevetoxin, known as brevenals, exhibit antagonistic properties and counteract the effects of the brevetoxins in neuronal and pulmonary model systems. Taken together, the inhalation toxicity of Florida red tides would appear to depend on the amount of each toxin present, as well as on the spectrum of molecular activities elicited by each toxin. Toxicity in a bloom is diminished by the amount brevenal present.
Figures

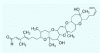
References
-
- Baden DG. 1977. Metabolism and Toxinology of Florida’s Red Tide Dinoflagellate Gymnodinium breve [PhD Thesis]. Miami, FL:University of Miami.
-
- Baden DG. Marine food-borne dinoflagellate toxins. Int Rev Cytol. 1983;82:99–150. - PubMed
-
- Baden, DG Biochemistry of the brevetoxins: potent activators of voltage-sensitive sodium channels. FASEB J. 1989;3:1807–1817. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

