The ATPase activity of BfpD is greatly enhanced by zinc and allosteric interactions with other Bfp proteins
- PMID: 15866879
- PMCID: PMC1224739
- DOI: 10.1074/jbc.M500253200
The ATPase activity of BfpD is greatly enhanced by zinc and allosteric interactions with other Bfp proteins
Retraction in
-
The ATPase activity of BfpD is greatly enhanced by zinc and allosteric interactions with other Bfp proteins.J Biol Chem. 2009 Jul 31;284(31):21100. doi: 10.1074/jbc.a500253200. J Biol Chem. 2009. PMID: 19785092 Free PMC article. No abstract available.
Abstract
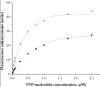
Type IV pilus biogenesis, protein secretion, DNA transfer, and filamentous phage morphogenesis systems are thought to possess similar architectures and mechanisms. These multiprotein complexes include members of the PulE superfamily of putative NTPases that have extensive sequence similarity and probably similar functions as the energizers of macromolecular transport. We purified the PulE homologue BfpD of the enteropathogenic Escherichia coli bundle-forming pilus (BFP) biogenesis machine and characterized its ATPase activity, providing new insights into its mode of action. Numerous techniques revealed that BfpD forms hexamers in the presence of nucleotide. Hexameric BfpD displayed weak ATPase activity. We previously demonstrated that the N termini of membrane proteins BfpC and BfpE recruit BfpD to the cytoplasmic membrane. Here, we identified two BfpD-binding sites, BfpE(39-76) and BfpE(77-114), in the N terminus of BfpE using a yeast two-hybrid system. Isothermal titration calorimetry and protease sensitivity assays showed that hexameric BfpD-ATPgammaS binds to BfpE(77-114), whereas hexameric BfpD-ADP binds to BfpE(39-76). Interestingly, the N terminus of BfpC and BfpE(77-114) together increased the ATPase activity of hexameric BfpD over 1200-fold to a V(max) of 75.3 mumol of P(i) min(-1) mg(-1), which exceeds by over 1200-fold the activity of other PulE family members. This augmented activity occurred only in the presence of Zn(2+). We conclude that allosteric interactions between BfpD and BfpC and BfpE dramatically stimulate its ATPase activity. The differential nucleotide-dependent binding of hexameric BfpD to BfpE(39-76) and BfpE(77-114) suggests a model for the mechanism by which BfpD transduces mechanical energy to the biogenesis machine.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

