STIM1, an essential and conserved component of store-operated Ca2+ channel function
- PMID: 15866891
- PMCID: PMC2171946
- DOI: 10.1083/jcb.200502019
STIM1, an essential and conserved component of store-operated Ca2+ channel function
Abstract
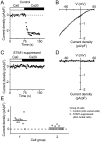
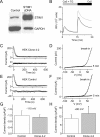
Store-operated Ca2+ (SOC) channels regulate many cellular processes, but the underlying molecular components are not well defined. Using an RNA interference (RNAi)-based screen to identify genes that alter thapsigargin (TG)-dependent Ca2+ entry, we discovered a required and conserved role of Stim in SOC influx. RNAi-mediated knockdown of Stim in Drosophila S2 cells significantly reduced TG-dependent Ca2+ entry. Patch-clamp recording revealed nearly complete suppression of the Drosophila Ca2+ release-activated Ca2+ (CRAC) current that has biophysical characteristics similar to CRAC current in human T cells. Similarly, knockdown of the human homologue STIM1 significantly reduced CRAC channel activity in Jurkat T cells. RNAi-mediated knockdown of STIM1 inhibited TG- or agonist-dependent Ca2+ entry in HEK293 or SH-SY5Y cells. Conversely, overexpression of STIM1 in HEK293 cells modestly enhanced TG-induced Ca2+ entry. We propose that STIM1, a ubiquitously expressed protein that is conserved from Drosophila to mammalian cells, plays an essential role in SOC influx and may be a common component of SOC and CRAC channels.
Figures
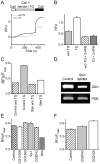
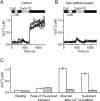
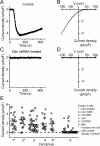
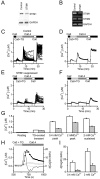


Comment in
-
Capacitative calcium entry: sensing the calcium stores.J Cell Biol. 2005 May 9;169(3):381-2. doi: 10.1083/jcb.200503161. Epub 2005 May 2. J Cell Biol. 2005. PMID: 15866892 Free PMC article.
References
-
- Clapham, D.E. 2003. TRP channels as cellular sensors. Nature. 426:517–524. - PubMed
-
- Cui, J., J.S. Bian, A. Kagan, and T.V. McDonald. 2002. CaT1 contributes to the store-operated calcium current in Jurkat T-lymphocytes. J. Biol. Chem. 277:47175–47183. - PubMed
-
- Fagan, K.A., K.E. Smith, and D.M. Cooper. 2000. Regulation of the Ca2+-inhibitable adenylyl cyclase type VI by capacitative Ca2+ entry requires localization in cholesterol-rich domains. J. Biol. Chem. 275:26530–26537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

