Defective O-antigen polymerization in tolA and pal mutants of Escherichia coli in response to extracytoplasmic stress
- PMID: 15866920
- PMCID: PMC1112028
- DOI: 10.1128/JB.187.10.3359-3368.2005
Defective O-antigen polymerization in tolA and pal mutants of Escherichia coli in response to extracytoplasmic stress
Abstract
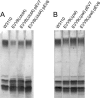
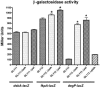
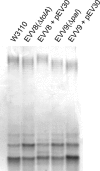
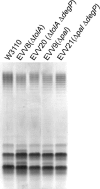
We have previously shown that the TolA protein is required for the correct surface expression of the Escherichia coli O7 antigen lipopolysaccharide (LPS). In this work, delta tolA and delta pal mutants of E. coli K-12 W3110 were transformed with pMF19 (encoding a rhamnosyltransferase that reconstitutes the expression of O16-specific LPS), pWQ5 (encoding the Klebsiella pneumoniae O1 LPS gene cluster), or pWQ802 (encoding the genes necessary for the synthesis of Salmonella enterica O:54). Both DeltatolA and delta pal mutants exhibited reduced surface expression of O16 LPS as compared to parental W3110, but no significant differences were observed in the expression of K. pneumoniae O1 LPS and S. enterica O:54 LPS. Therefore, TolA and Pal are required for the correct surface expression of O antigens that are assembled in a wzy (polymerase)-dependent manner (like those of E. coli O7 and O16) but not for O antigens assembled by wzy-independent pathways (like K. pneumoniae O1 and S. enterica O:54). Furthermore, we show that the reduced surface expression of O16 LPS in delta tolA and delta pal mutants was associated with a partial defect in O-antigen polymerization and it was corrected by complementation with intact tolA and pal genes, respectively. Using derivatives of W3110 delta tolA and W3110 delta pal containing lacZ reporter fusions to fkpA and degP, we also demonstrate that the RpoE-mediated extracytoplasmic stress response is upregulated in these mutants. Moreover, an altered O16 polymerization was also detected under conditions that stimulate RpoE-mediated extracytoplasmic stress responses in tol+ and pal+ genetic backgrounds. A Wzy derivative with an epitope tag at the C-terminal end of the protein was stable in all the mutants, ruling out stress-mediated proteolysis of Wzy. We conclude that the absence of TolA and Pal elicits a sustained extracytoplasmic stress response that in turn reduces O-antigen polymerization but does not affect the stability of the Wzy O-antigen polymerase.
Figures






Similar articles
-
Surface expression of O-specific lipopolysaccharide in Escherichia coli requires the function of the TolA protein.Mol Microbiol. 2000 Oct;38(2):262-75. doi: 10.1046/j.1365-2958.2000.02094.x. Mol Microbiol. 2000. PMID: 11069653
-
Energy-dependent conformational change in the TolA protein of Escherichia coli involves its N-terminal domain, TolQ, and TolR.J Bacteriol. 2001 Jul;183(14):4110-4. doi: 10.1128/JB.183.14.4110-4114.2001. J Bacteriol. 2001. PMID: 11418549 Free PMC article.
-
Deletion analyses of the peptidoglycan-associated lipoprotein Pal reveals three independent binding sequences including a TolA box.Mol Microbiol. 2004 Feb;51(3):873-85. doi: 10.1046/j.1365-2958.2003.03881.x. Mol Microbiol. 2004. PMID: 14731286
-
The Tol proteins of Escherichia coli and their involvement in the uptake of biomolecules and outer membrane stability.FEMS Microbiol Lett. 1999 Aug 15;177(2):191-7. doi: 10.1111/j.1574-6968.1999.tb13731.x. FEMS Microbiol Lett. 1999. PMID: 10474183 Review.
-
Colicin A binds to a novel binding site of TolA in the Escherichia coli periplasm.Biochem Soc Trans. 2012 Dec 1;40(6):1469-74. doi: 10.1042/BST20120239. Biochem Soc Trans. 2012. PMID: 23176500 Review.
Cited by
-
Comparative Analysis of Outer Membrane Vesicle Isolation Methods With an Escherichia coli tolA Mutant Reveals a Hypervesiculating Phenotype With Outer-Inner Membrane Vesicle Content.Front Microbiol. 2021 Mar 5;12:628801. doi: 10.3389/fmicb.2021.628801. eCollection 2021. Front Microbiol. 2021. PMID: 33746922 Free PMC article.
-
A snapshot of the λ T4rII exclusion (Rex) phenotype in Escherichia coli.Curr Genet. 2021 Oct;67(5):739-745. doi: 10.1007/s00294-021-01183-2. Epub 2021 Apr 20. Curr Genet. 2021. PMID: 33877398 Review.
-
CTXφ Replication Depends on the Histone-Like HU Protein and the UvrD Helicase.PLoS Genet. 2015 May 20;11(5):e1005256. doi: 10.1371/journal.pgen.1005256. eCollection 2015 May. PLoS Genet. 2015. PMID: 25992634 Free PMC article.
-
Interplay of the Wzx translocase and the corresponding polymerase and chain length regulator proteins in the translocation and periplasmic assembly of lipopolysaccharide o antigen.J Bacteriol. 2006 Jul;188(14):5124-35. doi: 10.1128/JB.00461-06. J Bacteriol. 2006. PMID: 16816184 Free PMC article.
-
Genes required for growth at high hydrostatic pressure in Escherichia coli K-12 identified by genome-wide screening.PLoS One. 2013 Sep 11;8(9):e73995. doi: 10.1371/journal.pone.0073995. eCollection 2013. PLoS One. 2013. PMID: 24040140 Free PMC article.
References
-
- Alba, B. M., and C. A. Gross. 2004. Regulation of the Escherichia coli sigma-dependent envelope stress response. Mol. Microbiol. 52:613-619. - PubMed
-
- Amer, A. O., and M. A. Valvano. 2002. Conserved aspartic acids are essential for the enzymic activity of the WecA protein initiating the biosynthesis of O-specific lipopolysaccharide and enterobacterial common antigen in Escherichia coli. Microbiology 148:571-582. - PubMed
-
- Bouveret, E., R. Derouiche, A. Rigal, R. Lloubes, C. Lazdunski, and H. Benedetti. 1995. Peptidoglycan-associated lipoprotein-TolB interaction. A possible key to explaining the formation of contact sites between the inner and outer membranes of Escherichia coli. J. Biol. Chem. 270:11071-11077. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

