Localization of chaperones DnaK and GroEL in bacterial inclusion bodies
- PMID: 15866952
- PMCID: PMC1111989
- DOI: 10.1128/JB.187.10.3599-3601.2005
Localization of chaperones DnaK and GroEL in bacterial inclusion bodies
Abstract
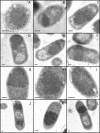
By immunostaining and transmission electron microscopy, chaperones DnaK and GroEL have been identified at the solvent-exposed surface of bacterial inclusion bodies and entrapped within these aggregates, respectively. Functional implications of this distinct localization are discussed in the context of Escherichia coli protein quality control.
Figures
References
-
- Baneyx, F., and M. Mujacic. 2004. Recombinant protein folding and misfolding in Escherichia coli. Nat. Biotechnol. 22:1399-1408. - PubMed
-
- Boels, K., M. M. Carrió, A. Arís, J. L. Corchero, and A. Villaverde. 1999. Distinct chaperone affinity to folding variants of homologous recombinant proteins. Biotechnol. Lett. 21:531-536.
-
- Carrio, M. M., J. L. Corchero, and A. Villaverde. 1998. Dynamics of in vivo protein aggregation: building inclusion bodies in recombinant bacteria. FEMS Microbiol. Lett. 169:9-15. - PubMed
-
- Carrio, M. M., J. L. Corchero, and A. Villaverde. 1999. Proteolytic digestion of bacterial inclusion body proteins during dynamic transition between soluble and insoluble forms. Biochim. Biophys. Acta 1434:170-176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

