Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse
- PMID: 15867092
- PMCID: PMC2213197
- DOI: 10.1084/jem.20042224
Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse
Abstract
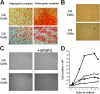
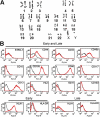
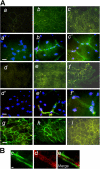
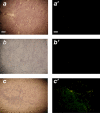
Here, we report the isolation of a human multipotent adipose-derived stem (hMADS) cell population from adipose tissue of young donors. hMADS cells display normal karyotype; have active telomerase; proliferate >200 population doublings; and differentiate into adipocytes, osteoblasts, and myoblasts. Flow cytometry analysis indicates that hMADS cells are CD44+, CD49b+, CD105+, CD90+, CD13+, Stro-1(-), CD34-, CD15-, CD117-, Flk-1(-), gly-A(-), CD133-, HLA-DR(-), and HLA-I(low). Transplantation of hMADS cells into the mdx mouse, an animal model of Duchenne muscular dystrophy, results in substantial expression of human dystrophin in the injected tibialis anterior and the adjacent gastrocnemius muscle. Long-term engraftment of hMADS cells takes place in nonimmunocompromised animals. Based on the small amounts of an easily available tissue source, their strong capacity for expansion ex vivo, their multipotent differentiation, and their immune-privileged behavior, our results suggest that hMADS cells will be an important tool for muscle cell-mediated therapy.
Figures

References
-
- Verfaillie, C.M. 2002. Adult stem cells: assessing the case for pluripotency. Trends Cell Biol. 12:502–508. - PubMed
-
- Pittenger, M.F., A.M. Mackay, S.C. Beck, R.K. Jaiswal, R. Douglas, J.D. Mosca, M.A. Moorman, D.W. Simonetti, S. Craig, and D.R. Marshak. 1999. Multilineage potential of adult human mesenchymal stem cells. Science. 284:143–147. - PubMed
-
- Krause, D.S., N.D. Theise, M.I. Collector, O. Henegariu, S. Hwang, R. Gardner, S. Neutzel, and S.J. Sharkis. 2001. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 105:369–377. - PubMed
-
- Jiang, Y., B. Vaessen, T. Lenvik, M. Blackstad, M. Reyes, and C.M. Verfaillie. 2002. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle and brain. Exp. Hematol. 30:896–904. - PubMed
-
- Jiang, Y., B.N. Jahagirdar, R.L. Reinhardt, R.E. Schwartz, C.D. Keene, X.R. Ortiz-Gonzalez, M. Reyes, T. Lenvik, T. Lund, M. Blackstad, et al. 2002. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 118:41–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

