Cyclooxygenase-1 is a potential target for prevention and treatment of ovarian epithelial cancer
- PMID: 15867369
- PMCID: PMC2584020
- DOI: 10.1158/0008-5472.CAN-04-3814
Cyclooxygenase-1 is a potential target for prevention and treatment of ovarian epithelial cancer
Abstract
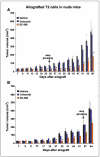
The precise genetic and molecular defects underlying epithelial ovarian cancer (EOC) remain largely unknown, and treatment options for patients with advanced disease are limited. Cyclooxygenases (COX-1 and COX-2) catalyze the conversion of arachidonic acid to prostaglandins. Whereas overwhelming evidence suggests a role for COX-2 in a variety of cancers, the contribution of COX-1 remains much less explored. The expression status of COX isoforms in ovarian cancers also remains confusing. We have previously shown that human epithelial ovarian tumors have increased levels of COX-1 but not COX-2. To more carefully examine the role of COXs in ovarian cancer, we used a mouse model of EOC in which genetic and oncogenic modifications were experimentally engineered into ovarian surface epithelial cells (OSE) thought to be the cells of origin for human EOC. These OSE cells produce tumors when allografted into host mice. Using multiple approaches, we observed that OSE cells and the tumors comprised of these cells express high levels of COX-1 but not COX-2. Prostacyclin (PGI(2)) is the major prostaglandin generated downstream of COX-1 in these cells, and SC-560, a COX-1-selective inhibitor, dramatically inhibits PGI(2) production. More importantly, SC-560 reduced the growth of tumors when OSE cells were allografted in nude female mice. In contrast, the COX-2-selective inhibitor celecoxib had little effect on tumor growth. The growth inhibitory effects of SC-560 result from reduced cell proliferation and/or accelerated apoptosis. Our results imply COX-1 as a target for the prevention and/or treatment of EOC.
Figures




Similar articles
-
Extracellular signal-regulated kinase is a target of cyclooxygenase-1-peroxisome proliferator-activated receptor-delta signaling in epithelial ovarian cancer.Cancer Res. 2007 Jun 1;67(11):5285-92. doi: 10.1158/0008-5472.CAN-07-0828. Cancer Res. 2007. PMID: 17545608
-
Inhibition of cyclooxygenase-1 and -2 expression by targeting the endothelin a receptor in human ovarian carcinoma cells.Clin Cancer Res. 2004 Jul 15;10(14):4670-9. doi: 10.1158/1078-0432.CCR-04-0315. Clin Cancer Res. 2004. PMID: 15269139
-
Direct evidence for a role of cyclooxygenase 2-derived prostaglandin E2 in human head and neck xenograft tumors.Cancer Res. 2002 Nov 15;62(22):6706-11. Cancer Res. 2002. PMID: 12438270
-
The role of COX-2 inhibition in breast cancer treatment and prevention.Semin Oncol. 2004 Apr;31(2 Suppl 7):22-9. doi: 10.1053/j.seminoncol.2004.03.042. Semin Oncol. 2004. PMID: 15179621 Review.
-
Dual acting anti-inflammatory drugs: a reappraisal.Pharmacol Res. 2001 Dec;44(6):437-50. doi: 10.1006/phrs.2001.0872. Pharmacol Res. 2001. PMID: 11735348 Review.
Cited by
-
Effects of a selective cyclooxygenase-1 inhibitor in SKOV-3 ovarian carcinoma xenograft-bearing mice.Med Oncol. 2010 Mar;27(1):98-104. doi: 10.1007/s12032-009-9179-y. Epub 2009 Feb 24. Med Oncol. 2010. PMID: 19235530
-
Circulating Prostaglandin Biosynthesis in Colorectal Cancer and Potential Clinical Significance.EBioMedicine. 2015 Feb 1;2(2):165-171. doi: 10.1016/j.ebiom.2014.12.004. EBioMedicine. 2015. PMID: 25750933 Free PMC article.
-
Effect of the combination of a cyclooxygenase-1 selective inhibitor and taxol on proliferation, apoptosis and angiogenesis of ovarian cancer in vivo.Oncol Lett. 2012 Jul;4(1):168-174. doi: 10.3892/ol.2012.688. Epub 2012 Apr 23. Oncol Lett. 2012. PMID: 22807982 Free PMC article.
-
The effects of the histone deacetylase inhibitor romidepsin (FK228) are enhanced by aspirin (ASA) in COX-1 positive ovarian cancer cells through augmentation of p21.Cancer Biol Ther. 2010 Jun 1;9(11):928-35. doi: 10.4161/cbt.9.11.11873. Epub 2010 Jun 25. Cancer Biol Ther. 2010. PMID: 20404564 Free PMC article.
-
Prostacyclin Released by Cancer-Associated Fibroblasts Promotes Immunosuppressive and Pro-Metastatic Macrophage Polarization in the Ovarian Cancer Microenvironment.Cancers (Basel). 2022 Dec 13;14(24):6154. doi: 10.3390/cancers14246154. Cancers (Basel). 2022. PMID: 36551640 Free PMC article.
References
-
- Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–60. - PubMed
-
- Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1:11–21. - PubMed
-
- Cramer DW, Harlow BL, Titus-Ernstoff L, Bohlke K, Welch WR, Greenberg ER. Over-the-counter analgesics and risk of ovarian cancer. Lancet. 1998;351:104–7. - PubMed
-
- Moysich KB, Mettlin C, Piver MS, Natarajan N, Menezes RJ, Swede H. Regular use of analgesic drugs and ovarian cancer risk. Cancer Epidemiol Biomarkers Prev. 2001;10:903–6. - PubMed
-
- Akhmedkhanov A, Toniolo P, Zeleniuch-Jacquotte A, Kato I, Koenig KL, Shore RE. Aspirin and epithelial ovarian cancer. Prev Med. 2001;33:682–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

