Chemical synthesis of proteins
- PMID: 15869385
- PMCID: PMC2845543
- DOI: 10.1146/annurev.biophys.34.040204.144700
Chemical synthesis of proteins
Abstract
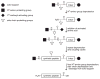
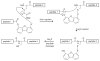
Proteins have become accessible targets for chemical synthesis. The basic strategy is to use native chemical ligation, Staudinger ligation, or other orthogonal chemical reactions to couple synthetic peptides. The ligation reactions are compatible with a variety of solvents and proceed in solution or on a solid support. Chemical synthesis enables a level of control on protein composition that greatly exceeds that attainable with ribosome-mediated biosynthesis. Accordingly, the chemical synthesis of proteins is providing previously unattainable insight into the structure and function of proteins.
Figures



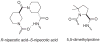
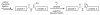









References
-
- Aimoto S. Polypeptide synthesis by the thioester method. Biopolymers. 1999;51:247–65. - PubMed
-
- Albericio F. Developments in peptide and amide synthesis. Curr Opin Chem Biol. 2004;8:211–21. - PubMed
-
- Albericio F, Carpino LA. Coupling reagents and activation. Methods Enzymol. 1997;289:104–26. - PubMed
-
- Albericio F, Lloyd-Williams P, Giralt E. Convergent solid-phase peptide synthesis. Methods Enzymol. 1997;289:313–36. - PubMed
-
- Arner ES, Sarioglu H, Lottspeich F, Holmgren A, Bock A. High-level expression in Escherichia coli of selenocysteine-containing rat thioredoxin reductase utilizing gene fusions with engineered bacterial-type SECIS elements and co-expression with the selA, selB and selC genes. J Mol Biol. 1999;292:1003–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
