Blockade of melanocortin transmission inhibits cocaine reward
- PMID: 15869520
- PMCID: PMC2694749
- DOI: 10.1111/j.1460-9568.2005.04038.x
Blockade of melanocortin transmission inhibits cocaine reward
Abstract
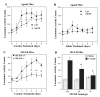
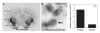
Melanocortins and the melanocortin-4 receptor (MC4-R) are enriched in the nucleus accumbens, a brain region that has been implicated in the rewarding action of cocaine and other drugs of abuse. In the present study we use a number of rat behavioral models to show that infusion of a melanocortin peptide antagonist into the nucleus accumbens blocks the reinforcing, incentive motivational, and locomotor sensitizing effects of cocaine. We also show that locomotor responses to repeated cocaine exposure are completely blocked in MC4-R null mutant mice and reduced in Agouti mice that overexpress an endogenous inhibitor of melanocortins in the brain. The results also demonstrate that cocaine administration increases the expression of MC4-R in the nucleus accumbens and striatum, and that MC4-R is co-localized with prodynorphin in medium spiny neurons in the nucleus accumbens. Together, these findings indicate that the behavioral actions of cocaine are dependent on activation of MC4-R, and suggest that upregulation of this receptor by drug exposure may contribute to sensitization of these behavioral responses. Modulation of cocaine reward is a novel action of the melanocortin-MC4-R system and could be targeted for the development of new medications for cocaine addiction.
Figures





References
-
- Adan R, Gispen WH. Brain melanocortin receptors: from cloning to function. Peptides. 1997;18:1279–1287. - PubMed
-
- Alvaro JD, Hsu R, Duman RS. Chronic cocaine administration increases the expression of MC4-R in rat neostriatum. J. Pharmacol. Exp. Ther. 2003;304:391–399. - PubMed
-
- Alvaro J, Tatro JB, Duman RS. Melanocortins and opiate addiction. Life Sci. 1997;61:1–9. - PubMed
-
- Alvaro J, Tatro JB, Quillan JM, Fogliano M, Eisenhard M, Lerner MR, Nestler EJ, Duman RS. Morphine down-regulates melanocortin-4 receptor expression in brain regions that mediate opiate addiction. Mol. Pharmacol. 1996;50:583–591. - PubMed
-
- de Vaca S. Cabeza, Kim G-Y, Carr KD. The melanocortin receptor agonist MTII augments the rewarding effect of amphetamine ad-libitum-fed and food-restricted rats. Psychopharmacol. 2002;161:77–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

