Evolution of temporal order in living organisms
- PMID: 15869714
- PMCID: PMC1142335
- DOI: 10.1186/1740-3391-3-7
Evolution of temporal order in living organisms
Abstract
Circadian clocks are believed to have evolved in parallel with the geological history of the earth, and have since been fine-tuned under selection pressures imposed by cyclic factors in the environment. These clocks regulate a wide variety of behavioral and metabolic processes in many life forms. They enhance the fitness of organisms by improving their ability to efficiently anticipate periodic events in their external environments, especially periodic changes in light, temperature and humidity. Circadian clocks provide fitness advantage even to organisms living under constant conditions, such as those prevailing in the depth of oceans or in subterranean caves, perhaps by coordinating several metabolic processes in the internal milieu. Although the issue of adaptive significance of circadian rhythms has always remained central to circadian biology research, it has never been subjected to systematic and rigorous empirical validation. A few studies carried out on free-living animals under field conditions and simulated periodic and aperiodic conditions of the laboratory suggest that circadian rhythms are of adaptive value to their owners. However, most of these studies suffer from a number of drawbacks such as lack of population-level replication, lack of true controls and lack of adequate control on the genetic composition of the populations, which in many ways limits the potential insights gained from the studies. The present review is an effort to critically discuss studies that directly or indirectly touch upon the issue of adaptive significance of circadian rhythms and highlight some shortcomings that should be avoided while designing future experiments.
Figures
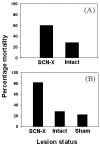
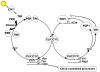

References
-
- DeCoursey PJ. The behavioral ecology and evolution of biological timing systems. In: Dunlap JC, Loros JJ, DeCoursey PJ, editor. Chronobiology: Biological Timekeeping. Sunderland, Massachusetts, USA:Sinauer Associates, Inc. Publishers; 2004. pp. 27–66.
-
- Sharma VK, Chandrashekaran MK. Zeitgebers (Time cues) for biological clocks. Current Sci.
LinkOut - more resources
Full Text Sources

