The nuclear import of TAF10 is regulated by one of its three histone fold domain-containing interaction partners
- PMID: 15870280
- PMCID: PMC1087738
- DOI: 10.1128/MCB.25.10.4092-4104.2005
The nuclear import of TAF10 is regulated by one of its three histone fold domain-containing interaction partners
Abstract
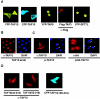
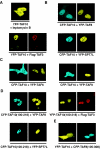
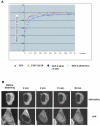
TFIID, comprising the TATA box binding protein (TBP) and 13 TBP-associated factors (TAFs), plays a role in nucleation in the assembly of the RNA polymerase II preinitiation complexes on protein-encoding genes. TAFs are shared among other transcription regulatory complexes (e.g., SAGA, TBP-free TAF-containing complex [TFTC], STAGA, and PCAF/GCN5). Human TAF10, a subunit of both TFIID and TFTC, has three histone fold-containing interaction partners: TAF3, TAF8, and SPT7Like (SPT7L). In human cells, exogenously expressed TAF10 remains rather cytoplasmic and leptomycin B does not affect this localization. By using fluorescent fusion proteins, we show that TAF10 does not have an intrinsic nuclear localization signal (NLS) and needs one of its three interaction partners to be transported into the nucleus. When the NLS sequences of either TAF8 or SPT7L are mutated, TAF10 remains cytoplasmic, but a heterologous NLS can drive TAF10 into the nucleus. Experiments using fluorescence recovery after photobleaching show that TAF10 does not associate with any cytoplasmic partner but that once transported into the nucleus it binds to nuclear structures. TAF10 binding to importin beta in vitro is dependent on the coexpression of either TAF8 or TAF3, but not SPT7L. The cytoplasmic-nuclear transport of TAF10 is naturally observed during the differentiation of adult male germ cells. Thus, here we describe a novel role of the three mammalian interacting partners in the nuclear localization of TAF10, and our data suggest that a complex network of regulated cytoplasmic associations may exist among these factors and that this network is important for the composition of different TFIID and TFTC-type complexes in the nucleus.
Figures





References
-
- Albright, S. R., and R. Tjian. 2000. TAFs revisited: more data reveal new twists and confirm old ideas. Gene 242:1-13. - PubMed
-
- Bell, B., E. Scheer, and L. Tora. 2001. Identification of hTAF(II)80 delta links apoptotic signaling pathways to transcription factor TFIID function. Mol. Cell 8:591-600. - PubMed
-
- Bell, B., and L. Tora. 1999. Regulation of gene expression by multiple forms of TFIID and other novel TAFII-containing complexes. Exp. Cell Res. 246:11-19. - PubMed
-
- Brand, M., C. Leurent, V. Mallouh, L. Tora, and P. Schultz. 1999. Three-dimensional structures of the TAFII-containing complexes TFIID and TFTC. Science 286:2151-2153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
