Balamuthia mandrillaris, free-living ameba and opportunistic agent of encephalitis, is a potential host for Legionella pneumophila bacteria
- PMID: 15870307
- PMCID: PMC1087515
- DOI: 10.1128/AEM.71.5.2244-2249.2005
Balamuthia mandrillaris, free-living ameba and opportunistic agent of encephalitis, is a potential host for Legionella pneumophila bacteria
Abstract
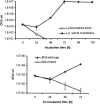
Balamuthia mandrillaris is a free-living ameba and an opportunistic agent of granulomatous encephalitis in humans and other mammalian species. Other free-living amebas, such as Acanthamoeba and Hartmannella, can provide a niche for intracellular survival of bacteria, including the causative agent of Legionnaires' disease, Legionella pneumophila. Infection of amebas by L. pneumophila enhances the bacterial infectivity for mammalian cells and lung tissues. Likewise, the pathogenicity of amebas may be enhanced when they host bacteria. So far, the colonization of B. mandrillaris by bacteria has not been convincingly shown. In this study, we investigated whether this ameba could host L. pneumophila bacteria. Our experiments showed that L. pneumophila could initiate uptake by B. mandrillaris and could replicate within the ameba about 4 to 5 log cycles from 24 to 72 h after infection. On the other hand, a dotA mutant, known to be unable to propagate in Acanthamoeba castellanii, also did not replicate within B. mandrillaris. Approaching completion of the intracellular cycle, L. pneumophila wild-type bacteria were able to destroy their ameboid hosts. Observations by light microscopy paralleled our quantitative data and revealed the rounding, collapse, clumping, and complete destruction of the infected amebas. Electron microscopic studies unveiled the replication of the bacteria in a compartment surrounded by a structure resembling rough endoplasmic reticulum. The course of intracellular infection, the degree of bacterial multiplication, and the ultrastructural features of a L. pneumophila-infected B. mandrillaris ameba resembled those described for other amebas hosting Legionella bacteria. We hence speculate that B. mandrillaris might serve as a host for bacteria in its natural environment.
Figures



References
-
- Amaral Zettler, L. A., T. A. Nerad, C. J. O'Kelly, M. T. Peglar, P. M. Gillevet, J. D. Silberman, and M. L. Sogin. 2000. A molecular reassessment of the leptomyxid amoebae. Protist 151:275-282. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

