Chemical forms of selenium in the metal-resistant bacterium Ralstonia metallidurans CH34 exposed to selenite and selenate
- PMID: 15870319
- PMCID: PMC1087582
- DOI: 10.1128/AEM.71.5.2331-2337.2005
Chemical forms of selenium in the metal-resistant bacterium Ralstonia metallidurans CH34 exposed to selenite and selenate
Abstract
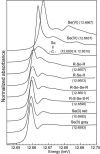
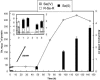
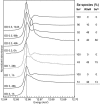
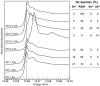
Ralstonia metallidurans CH34, a soil bacterium resistant to a variety of metals, is known to reduce selenite to intracellular granules of elemental selenium (Se(0)). We have studied the kinetics of selenite (Se(IV)) and selenate (Se(VI)) accumulation and used X-ray absorption spectroscopy to identify the accumulated form of selenate, as well as possible chemical intermediates during the transformation of these two oxyanions. When introduced during the lag phase, the presence of selenite increased the duration of this phase, as previously observed. Selenite introduction was followed by a period of slow uptake, during which the bacteria contained Se(0) and alkyl selenide in equivalent proportions. This suggests that two reactions with similar kinetics take place: an assimilatory pathway leading to alkyl selenide and a slow detoxification pathway leading to Se(0). Subsequently, selenite uptake strongly increased (up to 340 mg Se per g of proteins) and Se(0) was the predominant transformation product, suggesting an activation of selenite transport and reduction systems after several hours of contact. Exposure to selenate did not induce an increase in the lag phase duration, and the bacteria accumulated approximately 25-fold less Se than when exposed to selenite. Se(IV) was detected as a transient species in the first 12 h after selenate introduction, Se(0) also occurred as a minor species, and the major accumulated form was alkyl selenide. Thus, in the present experimental conditions, selenate mostly follows an assimilatory pathway and the reduction pathway is not activated upon selenate exposure. These results show that R. metallidurans CH34 may be suitable for the remediation of selenite-, but not selenate-, contaminated environments.
Figures

Similar articles
-
Mobilization of selenite by Ralstonia metallidurans CH34.Appl Environ Microbiol. 2001 Feb;67(2):769-73. doi: 10.1128/AEM.67.2.769-773.2001. Appl Environ Microbiol. 2001. PMID: 11157242 Free PMC article.
-
[Screening and identification of a photosynthetic bacterium reducing selenite to red elemental selenium].Wei Sheng Wu Xue Bao. 2007 Feb;47(1):44-7. Wei Sheng Wu Xue Bao. 2007. PMID: 17436622 Chinese.
-
Selenium bioaccumulation and speciation in Chironomus dilutus exposed to water-borne selenate, selenite, or seleno-DL-methionine.Environ Toxicol Chem. 2011 Oct;30(10):2292-9. doi: 10.1002/etc.624. Epub 2011 Aug 12. Environ Toxicol Chem. 2011. PMID: 21766323
-
Metabolic pathway for selenium in the body: speciation by HPLC-ICP MS with enriched Se.Food Addit Contam. 2002 Oct;19(10):974-83. doi: 10.1080/02652030210153578. Food Addit Contam. 2002. PMID: 12443560 Review.
-
[Progress in research of the product of the red elemental selenium reduced from selenium oxyanions by bacteria].Wei Sheng Wu Xue Bao. 2007 Jun;47(3):554-7. Wei Sheng Wu Xue Bao. 2007. PMID: 17672326 Review. Chinese.
Cited by
-
XANES measurements of the rate of radiation damage to selenomethionine side chains.J Synchrotron Radiat. 2007 Jan;14(Pt 1):51-72. doi: 10.1107/S0909049506048898. Epub 2006 Dec 15. J Synchrotron Radiat. 2007. PMID: 17211072 Free PMC article.
-
Microbial transformation of Se oxyanions in cultures of Delftia lacustris grown under aerobic conditions.J Microbiol. 2019 May;57(5):362-371. doi: 10.1007/s12275-019-8427-x. Epub 2019 Mar 21. J Microbiol. 2019. PMID: 30900147
-
Se(VI) reduction and the precipitation of Se(0) by the facultative bacterium Enterobacter cloacae SLD1a-1 are regulated by FNR.Appl Environ Microbiol. 2007 Mar;73(6):1914-20. doi: 10.1128/AEM.02542-06. Epub 2007 Jan 19. Appl Environ Microbiol. 2007. PMID: 17261520 Free PMC article.
-
Complete Genome Sequence of Bacillus cereus CC-1, A Novel Marine Selenate/Selenite Reducing Bacterium Producing Metallic Selenides Nanomaterials.Curr Microbiol. 2019 Jan;76(1):78-85. doi: 10.1007/s00284-018-1587-9. Epub 2018 Oct 20. Curr Microbiol. 2019. PMID: 30343326
-
Accumulation and metabolism of selenium by yeast cells.Appl Microbiol Biotechnol. 2015 Jul;99(13):5373-82. doi: 10.1007/s00253-015-6650-x. Epub 2015 May 24. Appl Microbiol Biotechnol. 2015. PMID: 26003453 Free PMC article. Review.
References
-
- Avazéri, C., R. Turner, J. Pommier, J. Weiner, G. Giordano, and A. Verméglio. 1997. Tellurite reductase activity of nitrate reductase is responsible for the basal resistance of Escherichia coli to tellurite. Microbiology 143:1181-1189. - PubMed
-
- Avoscan, L., M. Carrière, F. Jehanneuf, R. Collins, F. Carrot, J. Covès, and B. Gouget. 2004. Ralstonia metallidurans CH34 resistance to selenium oxyanions: growth kinetics, bioaccumulation and reduction, p. 267-271. In J. A. Centeno, P. Collery, and G. Vernet (ed.), Metal ions in biology and medicine, vol. 8. John Libbey Eurotext, Montrouge, France.
-
- Barkay, T., S. Miller, and A. Summers. 2003. Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiol. Rev. 27:355-384. - PubMed
-
- Bébien, M., J. Kirsch, V. Méjean, and A. Verméglio. 2002. Involvement of a putative molybdenum enzyme in the reduction of selenate by Escherichia coli. Microbiology 148:3865-3872. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

