Salvage chemotherapy with irinotecan, 5-fluorouracil and leucovorin for taxane- and cisplatin-refractory, metastatic gastric cancer
- PMID: 15870718
- PMCID: PMC2361777
- DOI: 10.1038/sj.bjc.6602575
Salvage chemotherapy with irinotecan, 5-fluorouracil and leucovorin for taxane- and cisplatin-refractory, metastatic gastric cancer
Abstract
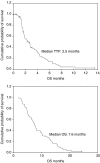
We performed a phase II study of combination chemotherapy with irinotecan, 5-fluorouracil (5-FU) and leucovorin in metastatic gastric cancer patients who were previously treated with taxane and cisplatin, to evaluate the antitumour activity and toxicity of the combination chemotherapy. The metastatic gastric adenocarcinoma patients who were previously treated with taxane and cisplatin combination as first line, and had at least one measurable lesion, 0-2 ECOG performance status and adequate organ functions, were considered eligible. They received irinotecan (150 mg m(-2), day 1) and leucovorin (100 mg m(-2), day 1), followed by continuous infusion of 5-FU (1000 mg m(-2) day(-1), days 1 and 2) every 2 weeks. Treatment was continued until progression of disease was observed. In all, 64 patients were treated with this combination chemotherapy. The median age of the patients was 55 years (range, 33-74 years), and the median ECOG performance status was 1 (0-1, 61 (95%)). Out of 64 patients, 57 were assessable for response. Among 57 assessable patients, no complete response and 12 partial responses were observed (overall response rate, 21%; 95% confidence interval (CI), 10-32%). Stable disease was observed in 14 patients (25%) and progressive disease in 31 patients (54%). The median time to progression was 2.5 months (95% CI, 1.6-3.4) and the median overall survival since the start of the second-line modified FOLFIRI was 7.6 months (95% CI, 6.5-8.7). Grade 3-4 haematologic toxicities included neutropenia in seven patients (11%) and thrombocytopenia in five patients (8%). Grade 3-4 nonhaematologic toxicities included diarrhoea in two patients (3%) and vomiting in two patients (3%). There were no treatment-related deaths. The combination of irinotecan, 5-FU and leucovorin showed moderate activity and favourable toxicity profile as a second-line treatment in metastatic gastric cancer patients, who were previously treated with taxane and cisplatin.
Similar articles
-
Irinotecan combined with 5-fluorouracil and leucovorin third-line chemotherapy after failure of fluoropyrimidine, platinum, and taxane in gastric cancer: treatment outcomes and a prognostic model to predict survival.Gastric Cancer. 2013 Oct;16(4):581-9. doi: 10.1007/s10120-012-0227-5. Epub 2012 Dec 25. Gastric Cancer. 2013. PMID: 23266882
-
A phase II study of irinotecan with biweekly, low dose leucovorin and bolus and continuous infusion 5-fluorouracil (modified FOLFIRI) as first line therapy for patients with recurrent or metastatic gastric cancer.Am J Clin Oncol. 2010 Jun;33(3):246-50. doi: 10.1097/COC.0b013e3181a650d4. Am J Clin Oncol. 2010. PMID: 19770628 Clinical Trial.
-
A phase II study of irinotecan, continuous 5-fluorouracil, and leucovorin (FOLFIRI) combination chemotherapy for patients with recurrent or metastatic gastric cancer previously treated with a fluoropyrimidine-based regimen.Am J Clin Oncol. 2010 Dec;33(6):572-6. doi: 10.1097/COC.0b013e3181bead7b. Am J Clin Oncol. 2010. PMID: 20042971 Clinical Trial.
-
Salvage chemotherapy with irinotecan and cisplatin in patients with metastatic gastric cancer failing both 5-fluorouracil and taxanes.Anticancer Drugs. 2005 Jul;16(6):621-5. doi: 10.1097/00001813-200507000-00005. Anticancer Drugs. 2005. PMID: 15930889 Clinical Trial.
-
Modified FOLFOX-6 therapy for heavily pretreated advanced gastric cancer refractory to fluorouracil, irinotecan, cisplatin and taxanes: a retrospective study.Jpn J Clin Oncol. 2012 Aug;42(8):686-90. doi: 10.1093/jjco/hys084. Epub 2012 Jun 11. Jpn J Clin Oncol. 2012. PMID: 22693245
Cited by
-
A Phase II study of cetuximab (Erbitux) plus FOLFIRI for irinotecan and oxaliplatin-refractory metastatic colorectal cancer.J Korean Med Sci. 2007 Sep;22 Suppl(Suppl):S98-S103. doi: 10.3346/jkms.2007.22.S.S98. J Korean Med Sci. 2007. PMID: 17923763 Free PMC article. Clinical Trial.
-
A pilot study of cisplatin, irinotecan, leucovorin and 5-fluorouracil (PILF) combination chemotherapy for advanced gastric cancer.Cancer Res Treat. 2006;38(3):121-5. doi: 10.4143/crt.2006.38.3.121. Epub 2006 Jun 30. Cancer Res Treat. 2006. PMID: 19771271 Free PMC article.
-
Second-line treatment of metastatic gastric cancer: Current options and future directions.World J Gastroenterol. 2015 Nov 7;21(41):11621-35. doi: 10.3748/wjg.v21.i41.11621. World J Gastroenterol. 2015. PMID: 26556991 Free PMC article. Review.
-
Second-line chemotherapy for advanced gastric cancer in Korea.Gastric Cancer. 2012 Oct;15(4):345-54. doi: 10.1007/s10120-011-0114-5. Epub 2012 Mar 13. Gastric Cancer. 2012. PMID: 22410800 Review.
-
Combination chemotherapy with irinotecan and cisplatin in pretreated patients with unresectable or recurrent gastric cancer.Gastric Cancer. 2006;9(3):203-7. doi: 10.1007/s10120-006-0379-2. Gastric Cancer. 2006. PMID: 16952039
References
-
- Ajani JA, Baker J, Pisters PW, Ho L, Mansfield PF, Feig BW, Charnsangavej C (2002) CPT-11 plus cisplatin in patients with advanced, untreated gastric or gastroesophageal junction carcinoma: results of a phase II study. Cancer 94: 641–646 - PubMed
-
- Ajani JA, Cutsem E, Moiseyenko V, Tjulandin S, Fodor M, Majlis A, Boni C, Zuber E, Blattmann A (2003) Docetaxel, cisplatin, 5-fluorouracil compare to cisplatin (C) and 5-fluorouracil (F) for chemotherapy-naïve patients with metastatic or locally recurrent, unresectable gastric carcinoma (MGC): Interim results of a randomized phase β trial (V325). Proc Am Soc Clin Oncol 22, (abstr. 999) p249
-
- Assersohn L, Brown G, Cunningham D, Ward C, Oates J, Waters JS, Hill ME, Norman AR (2004) Phase II study of irinotecan and 5-fluorouracil/leucovorin in patients with primary refractory or relapsed advanced oesophageal and gastric carcinoma. Ann Oncol 15: 64–69 - PubMed
-
- Bae JM, Won WJ, Jung KW (2002) Annual report of the Korea Central Cancer Registry Program 2000: based on registered data from 131 hospitals. Cancer Res 34: 77–83 - PubMed
-
- Blanke CD, Haller DG, Benson AB, Rothenberg ML, Berlin J, Mori M, Hsieh YC, Miller LL (2001) A phase II study of irinotecan with 5-fluorouracil and leucovorin in patients with previously untreated gastric adenocarcinoma. Ann Oncol 12: 1575–1580 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical