Zoledronic acid significantly improves pain scores and quality of life in breast cancer patients with bone metastases: a randomised, crossover study of community vs hospital bisphosphonate administration
- PMID: 15870721
- PMCID: PMC2361764
- DOI: 10.1038/sj.bjc.6602551
Zoledronic acid significantly improves pain scores and quality of life in breast cancer patients with bone metastases: a randomised, crossover study of community vs hospital bisphosphonate administration
Abstract
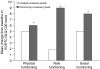
Patients with bone metastases from breast cancer often experience substantial skeletal complications -- including debilitating bone pain -- which negatively affect quality of life. Zoledronic acid (4 mg) has been demonstrated to reduce significantly the risk of skeletal complications in these patients and is administered via a short, 15-min infusion every 3 weeks, allowing the possibility for home administration. This study compared the efficacy and safety of zoledronic acid administered in the community setting vs the hospital setting in breast cancer patients with > or =1 bone metastasis receiving hormonal therapy. After a lead-in phase of three infusions of 4 mg zoledronic acid in the hospital setting, 101 patients were randomized to receive three open-label infusions in the community or hospital setting, followed by three infusions in the opposite venue (a total of nine infusions). The Brief Pain Inventory (BPI) and the European Organisation for Research and Treatment of Cancer Quality of Life Core Questionnaire 30 (EORTC QLQ-C30) were used to assess potential benefits of zoledronic acid therapy. At study end, analysis of the BPI showed significant reductions in worst pain (P=0.008) and average pain in the last 7 days (P=0.039), and interference with general activity (P=0.012). In each case, there were significantly greater improvements in pain scores after treatment in the community setting compared with the hospital crossover setting for worst pain (P=0.021), average pain (P=0.003), and interference with general activity (P=0.001). Overall global health status showed a significant median improvement of 8.3% (P=0.013) at study end. Physical, emotional, and social functioning also showed significant overall improvement (P=0.013, 0.005, and 0.043, respectively). Furthermore, physical, role, and social functioning showed significantly greater improvements after treatment in the community setting compared with the hospital crossover setting (P=0.018, 0.001, and 0.026, respectively). There was no difference between hospital and community administration in renal or other toxicity, with zoledronic acid being well tolerated in both treatment settings. These data confirm the safety and quality-of-life benefits of zoledronic acid in breast cancer patients with bone metastases, particularly when administered in the community setting.
Figures



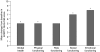
References
-
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85: 365–376 - PubMed
-
- Cleeland CS, Ryan KM (1994) Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 23: 129–138 - PubMed
-
- Coleman RE (2001) Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27: 165–176 - PubMed
-
- Department of Human Services (2003) Victoria's Hospital in the Home program. Victoria: State Government of Victoria
-
- Domchek SM, Younger J, Finkelstein DM, Seiden MV (2000) Predictors of skeletal complications in patients with metastatic breast carcinoma. Cancer 89: 363–368 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

