CREB/PKA sensitive signalling pathways activate and maintain expression levels of the hepatitis B virus pre-S2/S promoter
- PMID: 15871998
- PMCID: PMC1774645
- DOI: 10.1136/gut.2005.065086
CREB/PKA sensitive signalling pathways activate and maintain expression levels of the hepatitis B virus pre-S2/S promoter
Abstract
Background and aims: CREB (cAMP response element binding protein) transcription factors are key regulators of homeostatic functions in the liver, and CRE binding is increased in hepatic inflammation. During chronic hepatitis B virus (HBV) infection, mutations or deletions in the pre-S region are frequently observed. These mutations can affect the pre-S2/S promoter controlling HBV envelope protein expression (hepatitis B surface antigen (HBsAg)) and have been associated with worsened clinical outcome. We aimed to test if CREB activation impacts on HBsAg expression.
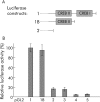
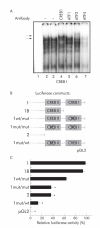
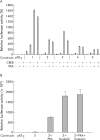
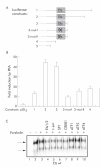
Methods: The effect of the CREB inducer protein kinase A (PKA) was tested by coexpression with HBV wild-type vector in vitro. Luciferase reporter gene constructs were cloned to identify novel regulatory regions for the HBV pre-S2/S promoter. Electrophoretic mobility shift assay (EMSA) gelshift and supershift experiments were conducted to confirm DNA transcription factor binding.
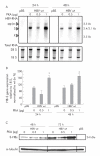
Results: Coexpression of HBV and PKA resulted in HBV-S mRNA induction and enhanced small envelope protein expression. We identified a CREB binding motif in the transcribed part of the pre-S2 region, contributing to basal S promoter activity via binding of activating transcription factor 2 (ATF2). A second CREB motif closely linked to the S-ATG showed a similar binding pattern involving ATF2 and CREB1, without appearing essential for basal promoter activity. Moreover, a sequence in the pre-S2 region is responsible for further transcriptional induction via CREB activators such as PKA and forskolin. EMSA experiments indicate that CREB1 and ATF4 are involved in complex formation conferring PKA dependent promoter activation.
Conclusions: Our data suggest a novel mechanism by which HBV may utilise CREB/PKA signal transduction pathways of hepatocytes to enhance its HBsAg expression during homeostasis and hepatic inflammation.
Figures


References
-
- Servillo G, Della Fazia MA, Sassone-Corsi P. Coupling cAMP signaling to transcription in the liver: pivotal role of CREB and CREM. Exp Cell Res 2002;275:143–54. - PubMed
-
- Herzig S, Hedrick S, Morantte I, et al. CREB controls hepatic lipid metabolism through nuclear hormone receptor PPAR-gamma. Nature 2003;426:190–3. - PubMed
-
- Herzig S, Long F, Jhala US, et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 2001;413:179–83. - PubMed
-
- Zhang B, Liu S, Perpetua MD, et al. Cytokines increase CRE binding but decrease CRE-mediated reporter activity in rat hepatocytes by increasing c-Jun. Hepatology 2004;39:1343–52. - PubMed
-
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol 2001;2:599–609. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
