Long-term self-renewal of postnatal muscle-derived stem cells
- PMID: 15872085
- PMCID: PMC1165414
- DOI: 10.1091/mbc.e05-02-0169
Long-term self-renewal of postnatal muscle-derived stem cells
Abstract
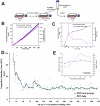
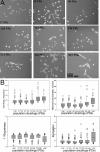
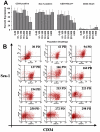
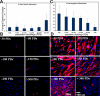
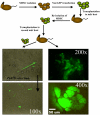
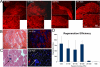
The ability to undergo self-renewal is a defining characteristic of stem cells. Self-replenishing activity sustains tissue homeostasis and regeneration. In addition, stem cell therapy strategies require a heightened understanding of the basis of the self-renewal process to enable researchers and clinicians to obtain sufficient numbers of undifferentiated stem cells for cell and gene therapy. Here, we used postnatal muscle-derived stem cells to test the basic biological assumption of unlimited stem cell replication. Muscle-derived stem cells (MDSCs) expanded for 300 population doublings (PDs) showed no indication of replicative senescence. MDSCs preserved their phenotype (ScaI+/CD34+/desmin(low)) for 200 PDs and were capable of serial transplantation into the skeletal muscle of mdx mice, which model Duchenne muscular dystrophy. MDSCs expanded to this level exhibited high skeletal muscle regeneration comparable with that exhibited by minimally expanded cells. Expansion beyond 200 PDs resulted in lower muscle regeneration, loss of CD34 expression, loss of myogenic activity, and increased growth on soft agar, suggestive of inevitable cell aging attributable to expansion and possible transformation of the MDSCs. Although these results raise questions as to whether cellular transformations derive from cell culturing or provide evidence of cancer stem cells, they establish the remarkable long-term self-renewal and regeneration capacity of postnatal MDSCs.
Figures



Similar articles
-
Nerve growth factor improves the muscle regeneration capacity of muscle stem cells in dystrophic muscle.Hum Gene Ther. 2006 Feb;17(2):180-92. doi: 10.1089/hum.2006.17.180. Hum Gene Ther. 2006. PMID: 16454652
-
NF-κB negatively impacts the myogenic potential of muscle-derived stem cells.Mol Ther. 2012 Mar;20(3):661-8. doi: 10.1038/mt.2011.261. Epub 2011 Dec 13. Mol Ther. 2012. PMID: 22158056 Free PMC article.
-
Pax7 is necessary and sufficient for the myogenic specification of CD45+:Sca1+ stem cells from injured muscle.PLoS Biol. 2004 May;2(5):E130. doi: 10.1371/journal.pbio.0020130. Epub 2004 May 11. PLoS Biol. 2004. PMID: 15138500 Free PMC article.
-
Muscle-derived stem cells: potential for muscle regeneration.Birth Defects Res C Embryo Today. 2003 Aug;69(3):230-7. doi: 10.1002/bdrc.10020. Birth Defects Res C Embryo Today. 2003. PMID: 14671776 Review.
-
Gene therapy and tissue engineering based on muscle-derived stem cells.Curr Opin Mol Ther. 2002 Aug;4(4):382-9. Curr Opin Mol Ther. 2002. PMID: 12222876 Review.
Cited by
-
NF-κB inhibition reveals a novel role for HGF during skeletal muscle repair.Cell Death Dis. 2015 Apr 23;6(4):e1730. doi: 10.1038/cddis.2015.66. Cell Death Dis. 2015. PMID: 25906153 Free PMC article.
-
Stem cell transplantation for treating Duchenne muscular dystrophy: A Web of Science-based literature analysis.Neural Regen Res. 2012 Aug 5;7(22):1744-51. doi: 10.3969/j.issn.1673-5374.2012.22.010. Neural Regen Res. 2012. PMID: 25624797 Free PMC article.
-
Dopaminergic neuronal conversion from adult rat skeletal muscle-derived stem cells in vitro.Neurochem Res. 2012 Sep;37(9):1982-92. doi: 10.1007/s11064-012-0819-9. Epub 2012 Jun 22. Neurochem Res. 2012. PMID: 22723079
-
An Estimation of Radiobiological Parameters for Head-and-Neck Cancer Cells and the Clinical Implications.Cancers (Basel). 2012 Jun 15;4(2):566-80. doi: 10.3390/cancers4020566. Cancers (Basel). 2012. PMID: 24213325 Free PMC article.
-
Applications of skeletal muscle progenitor cells for neuromuscular diseases.Am J Stem Cells. 2012 Nov 30;1(3):253-63. Print 2012. Am J Stem Cells. 2012. PMID: 23671812 Free PMC article.
References
-
- Amit, M., Carpenter, M. K., Inokuma, M. S., Chiu, C. P., Harris, C. P., Waknitz, M. A., Itskovitz-Eldor, J., and Thomson, J. A. (2000). Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev. Biol. 227, 271–278. - PubMed
-
- Berghella, L., et al. (1999). Reversible immortalization of human myogenic cells by site-specific excision of a retrovirally transferred oncogene. Hum. Gene Ther. 10, 1607–1617. - PubMed
-
- Bhardwaj, G., Murdoch, B., Wu, D., Baker, D. P., Williams, K. P., Chadwick, K., Ling, L. E., Karanu, F. N., and Bhatia, M. (2001). Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat. Immunol. 2, 172–180. - PubMed
-
- Bianchi, G., Banfi, A., Mastrogiacomo, M., Notaro, R., Luzzatto, L., Cancedda, R., and Quarto, R. (2003). Ex vivo enrichment of mesenchymal cell progenitors by fibroblast growth factor 2. Exp. Cell Res. 287, 98–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

