Maternal deprivation increases vulnerability to morphine dependence and disturbs the enkephalinergic system in adulthood
- PMID: 15872092
- PMCID: PMC6725024
- DOI: 10.1523/JNEUROSCI.4807-04.2005
Maternal deprivation increases vulnerability to morphine dependence and disturbs the enkephalinergic system in adulthood
Erratum in
- J Neurosci. 2005 Nun 22;25(25):6024
Abstract
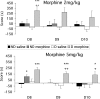
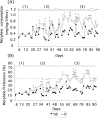
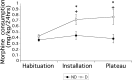
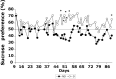
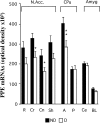
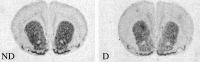
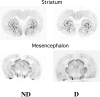
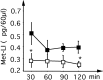
Maternal deprivation can trigger long-lasting molecular and cellular modifications in brain functions and might facilitate the appearance of pathogenic behaviors. This study focuses on the vulnerability to develop morphine dependence in adult rats that were separated from their mother and littermates for 3 h per day for 14 d after birth and examines the adaptive changes in the enkephalinergic pathways. Place-preference conditioning was observed with 2 mg/kg morphine in deprived rats, whereas 5 mg/kg morphine was necessary to induce conditioning in nondeprived animals. A prolonged morphine conditioning was shown in deprived rats. A strong increase in oral morphine self-administration behavior and preference was observed in deprived rats. Only a very slight increase in preference for sucrose solution, a more ethological reinforcer known to interact with the opioid system, was shown in deprived rats. These results indicate that this postnatal environment change leads to a hypersensitivity to the reinforcing properties of morphine and to the development of morphine dependence. A significant decrease in preproenkephalin mRNA expression was observed in the nucleus accumbens and the caudate-putamen nucleus of deprived rats. The basal extracellular levels of the Met-enkephalin-like immunoreactivity in the nucleus accumbens were significantly lower in deprived rats when compared with nondeprived animals, whereas no change in mu-opioid receptor binding occurred. These results strongly support that maternal deprivation leads to a basal hypoactivity of the enkephalinergic system and hypersensitivity to morphine effects. Together, our results suggest that maternal deprivation in pups likely represents a risk factor for morphine dependence in adult rats.
Figures

Similar articles
-
Adolescent exposure to chronic delta-9-tetrahydrocannabinol blocks opiate dependence in maternally deprived rats.Neuropsychopharmacology. 2009 Oct;34(11):2469-76. doi: 10.1038/npp.2009.70. Epub 2009 Jun 24. Neuropsychopharmacology. 2009. PMID: 19553915
-
Modulation of the endogenous opioid system after morphine self-administration and during its extinction: a study in Lewis and Fischer 344 rats.Neuropharmacology. 2007 Mar;52(3):931-48. doi: 10.1016/j.neuropharm.2006.10.011. Epub 2006 Dec 11. Neuropharmacology. 2007. PMID: 17161852
-
Chronic morphine treatment modulates the extracellular levels of endogenous enkephalins in rat brain structures involved in opiate dependence: a microdialysis study.J Neurosci. 2002 Feb 1;22(3):1034-41. doi: 10.1523/JNEUROSCI.22-03-01034.2002. J Neurosci. 2002. PMID: 11826132 Free PMC article.
-
Brief early handling increases morphine dependence in adult rats.Behav Brain Res. 2006 Jun 30;170(2):211-8. doi: 10.1016/j.bbr.2006.02.022. Epub 2006 Mar 29. Behav Brain Res. 2006. PMID: 16567006
-
Possible involvement of endocannabinoids in the increase of morphine consumption in maternally deprived rat.Neuropharmacology. 2013 Feb;65:193-9. doi: 10.1016/j.neuropharm.2012.10.008. Epub 2012 Oct 23. Neuropharmacology. 2013. PMID: 23089638
Cited by
-
Potentiation of glutamatergic synaptic transmission onto lateral habenula neurons following early life stress and intravenous morphine self-administration in rats.Addict Biol. 2022 Jan;27(1):e13064. doi: 10.1111/adb.13064. Epub 2021 May 25. Addict Biol. 2022. PMID: 34036710 Free PMC article.
-
Emotional valence and context of social influences on drug abuse-related behavior in animal models of social stress and prosocial interaction.Psychopharmacology (Berl). 2012 Nov;224(1):33-56. doi: 10.1007/s00213-012-2853-3. Epub 2012 Sep 7. Psychopharmacology (Berl). 2012. PMID: 22955569 Free PMC article. Review.
-
Traumatic Injury to the Developing Brain: Emerging Relationship to Early Life Stress.Front Neurol. 2021 Aug 18;12:708800. doi: 10.3389/fneur.2021.708800. eCollection 2021. Front Neurol. 2021. PMID: 34484104 Free PMC article. Review.
-
The effect of stress on opioid addiction-related behaviors: A review of preclinical literature.Exp Clin Psychopharmacol. 2023 Apr;31(2):523-540. doi: 10.1037/pha0000588. Epub 2022 Jul 14. Exp Clin Psychopharmacol. 2023. PMID: 35834183 Free PMC article. Review.
-
Early Life Stress and Risks for Opioid Misuse: Review of Data Supporting Neurobiological Underpinnings.J Pers Med. 2021 Apr 19;11(4):315. doi: 10.3390/jpm11040315. J Pers Med. 2021. PMID: 33921642 Free PMC article. Review.
References
-
- American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders, Ed 4, revised. Washington, DC: American Psychiatric Association.
-
- Anisman H, Zaharia MD, Meaney MJ, Meralis Z (1998) Do early-life events permanently alter behavioral and hormonal responses to stressors. Int J Dev Neurosci 16: 149-164. - PubMed
-
- Brady LS, Herkenham M, Rothman RB, Partilla JS, Kônig M, Zimmer AM, Zimmer A (1999) Region-specific up-regulation of opioid receptor binding in enkephalin knockout mice. Mol Brain Res 68: 193-197. - PubMed
-
- Caboche J, Vernier P, Julien JF, Rogard M, Mallet J, Besson M-J (1991) Parallel decrease of glutamic acid decarboxylase and preproenkephalin mRNA in the rat striatum following chronic treatment with a dopaminergic D1 antagonist and D2 agonist. J Neurochem 56: 428-435. - PubMed
-
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ (2000) The effects of early rearing environment on the development of GABAA and benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology 22: 219-229. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
