Locus ceruleus control of slow-wave homeostasis
- PMID: 15872097
- PMCID: PMC6725032
- DOI: 10.1523/JNEUROSCI.4845-04.2005
Locus ceruleus control of slow-wave homeostasis
Abstract
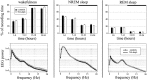
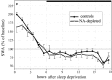
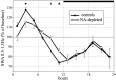
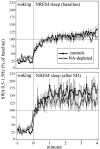
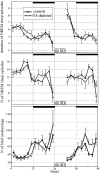
Sleep intensity is regulated by the duration of previous wakefulness, suggesting that waking results in the progressive accumulation of sleep need (Borbely and Achermann, 2000). In mammals, sleep intensity is reflected by slow-wave activity (SWA) in the nonrapid eye movement (NREM) sleep electroencephalogram, which increases in proportion to the time spent awake. However, the mechanisms responsible for the increase of NREM SWA after wakefulness remain unclear. According to a recent hypothesis (Tononi and Cirelli, 2003), the increase in SWA occurs because during wakefulness, many cortical circuits undergo synaptic potentiation, as evidenced by the widespread induction of long-term potentiation (LTP)-related genes in the brain of awake animals. A direct prediction of this hypothesis is that manipulations interfering with the induction of LTP-related genes should result in a blunted SWA response. Here, we examined SWA response in rats in which cortical norepinephrine (NA) was depleted, a manipulation that greatly reduces the induction of LTP-related genes during wakefulness (Cirelli and Tononi, 2004). We found that the homeostatic response of the lower-range SWA was markedly and specifically reduced after NA depletion. These data suggest that the wake-dependent accumulation of sleep need is causally related to cellular changes dependent on NA release, such as the induction of LTP-related genes, and support the hypothesis that sleep SWA homeostasis may be related to synaptic potentiation during wakefulness.
Figures


References
-
- Alanko LO, Laitinen JT, Stenberg D, Porkka-Heiskanen T (2004) Adenosine A1 receptor-dependent G-protein activity in the rat brain during prolonged wakefulness. NeuroReport 15: 2133-2137. - PubMed
-
- al-Zahrani SS, al-Ruwaitea AS, Ho MY, Bradshaw CM, Szabadi E (1997) Destruction of central noradrenergic neurones with DSP4 impairs the acquisition of temporal discrimination but does not affect memory for duration in a delayed conditional discrimination task. Psychopharmacology 130: 166-173. - PubMed
-
- Archer T, Mohammed AK, Ross SB, Soderberg U (1983) T-maze learning, spontaneous activity and food intake recovery following systemic administration of the noradrenaline neurotoxin, DSP4. Pharmacol Biochem Behav 19: 121-130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
