Short photoperiods impair spatial learning and alter hippocampal dendritic morphology in adult male white-footed mice (Peromyscus leucopus)
- PMID: 15872099
- PMCID: PMC6725029
- DOI: 10.1523/JNEUROSCI.0795-05.2005
Short photoperiods impair spatial learning and alter hippocampal dendritic morphology in adult male white-footed mice (Peromyscus leucopus)
Abstract
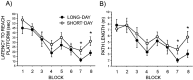
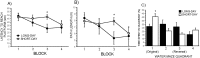
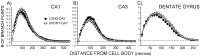
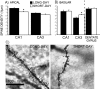
Although seasonal changes in brain morphology and function are well established in songbirds, seasonal plasticity of brain structure and function remain less well documented in mammals. Nontropical animals display many adaptations to reduce energy use to survive winter, including cessation of reproductive activities. Because of the high energetic costs of brain tissue, we hypothesized that male white-footed mice (Peromyscus leucopus) would reduce brain size in response to short days as well as regress their reproductive systems. Because short days may decrease hippocampal volume and impair spatial learning and memory in rodents and because of the potential for seasonal plasticity in the hippocampus, we hypothesized that photoperiod alters hippocampal morphology to affect spatial learning and memory. Mice housed in either long or short days for 10 weeks were examined for performance in a water maze; brains were then removed and weighed, and hippocampal volumes were determined. We also measured dendritic morphology and spine density in the CA1, CA3, and dentate gyrus. Short days decreased brain mass and hippocampal volume compared with long days. Short days also impaired long-term spatial learning and memory relative to long days but did not affect sensory discrimination or other types of memory. Short days decreased apical (stratum lacunosum-moleculare) CA1 spine density, as well as increased basilar (stratum oriens) CA3 spine density. Results from this study suggest that photoperiod alters brain size and morphology, as well as cognitive function. Understanding the mechanisms mediating these photoperiod-induced alterations may provide insight for treatment of seasonal cognitive and affective disorders.
Figures
References
-
- Brenowitz EA, Margoliash D, Nordeen KW (1997) An introduction to bird-song and the avian song system. J Neurobiol 33: 495-500. - PubMed
-
- Bronson FH (1985) Mammalian reproduction: an ecological perspective. Biol Reprod 32: 1-26. - PubMed
-
- Crawley JN (2000) What's wrong with my mouse? Behavioral phenotyping of transgenic and knockout mice. New York: Wiley.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
