LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing
- PMID: 15875025
- PMCID: PMC1369590
- DOI: 10.1038/nature03505
LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing
Abstract
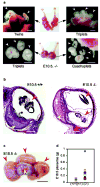
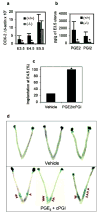
Every successful pregnancy requires proper embryo implantation. Low implantation rate is a major problem during infertility treatments using assisted reproductive technologies. Here we report a newly discovered molecular influence on implantation through the lysophosphatidic acid (LPA) receptor LPA3 (refs 2-4). Targeted deletion of LPA3 in mice resulted in significantly reduced litter size, which could be attributed to delayed implantation and altered embryo spacing. These two events led to delayed embryonic development, hypertrophic placentas shared by multiple embryos and embryonic death. An enzyme demonstrated to influence implantation, cyclooxygenase 2 (COX2) (ref. 5), was downregulated in LPA3-deficient uteri during pre-implantation. Downregulation of COX2 led to reduced levels of prostaglandins E2 and I2 (PGE2 and PGI2), which are critical for implantation. Exogenous administration of PGE2 or carbaprostacyclin (a stable analogue of PGI2) into LPA3-deficient female mice rescued delayed implantation but did not rescue defects in embryo spacing. These data identify LPA3 receptor-mediated signalling as having an influence on implantation, and further indicate linkage between LPA signalling and prostaglandin biosynthesis.
Conflict of interest statement
Figures


Comment in
-
Reproductive biology: fatty link to fertility.Nature. 2005 May 5;435(7038):34-5. doi: 10.1038/435034a. Nature. 2005. PMID: 15875005 No abstract available.
References
-
- Dey SK, et al. Molecular cues to implantation. Endocr Rev. 2004;25:341–73. - PubMed
-
- Ishii I, Fukushima N, Ye X, Chun J. LYSOPHOSPHOLIPID RECEPTORS: Signaling and Biology. Annu Rev Biochem. 2004;73:321–354. - PubMed
-
- Bandoh K, et al. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J Biol Chem. 1999;274:27776–85. - PubMed
-
- Contos JJ, Chun J. The mouse lp(A3)/Edg7 lysophosphatidic acid receptor gene: genomic structure, chromosomal localization, and expression pattern. Gene. 2001;267:243–53. - PubMed
-
- Lim H, et al. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

